Comprehensive Multilevel Characterization of Biologics Using Sheathless Capillary Electrophoresis Hyphenated to Tandem Mass Spectrometry
Special Issues
An analytical methodology for the characterization of the primary structure of biotherapeutic proteins using sheathless CE–ESI-MS-MS instrumentation is presented. For the first time, complete sequence coverage can be achieved using a bottom-up proteomic approach from a single analysis of a tryptic digest. In a biosimilarity assessment, a single amino acid substitution was detected.
An innovative coupling of capillary electrophoresis and tandem mass spectrometry (CE–MS-MS) provides excellent results for the characterization of the primary structure of biologics. Using a single 200-fmol injection, it is possible to completely characterize the amino acid sequence and obtain information about the location, structure, and relative abundance of 15 glycoforms. Finally, various post-translational modifications can be characterized and their relative abundances estimated, including asparagine deamidation and aspartic acid racemization. And in a biosimilarity assessment between two monoclonal antibodies, it was possible to distinguish minor differences such as a sole amino acid substitution. This level of characterization is permitted by cumulating the specificities of CE and high-resolution tandem MS using a sheathless interface.
The term biologics is often used to refer to biotherapeutic proteins in their various formats: monoclonal antibodies (mAbs), bispecific antibodies, fusion proteins, and more recently, antibody-drug conjugates. Biologics are currently having exceptional success in the pharmaceutical industry. The example of mAbs clearly illustrates this trend; mAbs are currently the fastest-growing category of therapeutic molecules in terms of market share (1). The enthusiasm for these therapeutics is explained by their mechanism of action, therapeutic potency, and specificity, which open new pathways for therapeutic treatment of complex diseases such as various forms of cancer or Crohn’s disease, for example. Additionally, their favorable pharmacodynamics and pharmacokinetics make them quite compatible for use in therapeutic treatments, and mAbs entering phase I clinical trials show a successful final approval rate of 23%, compared to the overall success rate, for all types of treatments, of only 10.4% (2,3). However, mAbs, like other types of biologics, are complex glycoproteins and may have a plethora of molecular microheterogeneities, in some cases introduced during the production process or long-term storage. Therefore, for safety reasons, regulatory agencies such as the U.S. Food and Drug Administration and the European Medicines Agency, require extensive characterization of these proteins and their microheterogeneities, some of which can influence the potency or efficacy of the protein. In addition, the recent introduction of biosimilar products raises many questions from regulatory agencies, which require that biopharmaceutical companies produce detailed analytical data to prove, as extensively as possible, the similarity between a biosimilar candidate and its already approved equivalent (4,5). In this regulatory context and given the structural complexity of biologics, their characterization represents an ongoing challenge for the analytical sciences, leading to the development of innovative analytical methodologies to address variability at a detailed level (6–8).
Mass spectrometry (MS) plays a significant role in mAb characterization, because of its excellent selectivity and sensitivity and its ability to provide structural information about an analyte. As a result, MS is currently used routinely in research and development (R&D) activities in the biopharmaceutical industry (9,10). Given the structural complexity of such therapeutic proteins, characterization must cover multiple aspects of the protein’s structure, such as amino acid sequence, disulfide bridges, glycosylation, and other post-translational modifications (PTMs). Such characterization requires the use of several orthogonal methods in combination with MS. Liquid chromatography (LC) is a primary technique, but the use of additional methods that improve the ability to obtain various levels of structural information simultaneously in a time-efficient and robust manner would be an eminent asset for improving R&D operations, and could potentially improve drug pipelines. Capillary electrophoresis (CE)–MS offers such potential. Although it was developed after the introduction of electrospray ionization (ESI) sources for MS (11), CE–MS tends to be used much less commonly than LC–MS.
Recently, our group developed an analytical method using capillary electrophoresis coupled to high-resolution tandem mass spectrometry (CE–MS-MS) for the characterization of mAbs. For these experiments, a novel sheathless CE–ESI-MS platform was used. The system implements a CE–ESI-MS interfacing developed by Sciex based on an original designed by Moini and colleagues (12). It does not rely on a sheath liquid to maintain the electrical field inside the CE capillary and provides an exquisite sensitivity (13). The biologic considered here is trastuzumab. Trastuzumab (sold by Genentech under the brand name “Herceptin”) is a monoclonal antibody currently approved for the treatment of HER-2 positive breast cancer. It has been extensively studied in recent years and is often used as a model mAb for the development and evaluation of new analytical methods for mAb characterization.
In our method, trastuzumab underwent proteolytic digestion by trypsin using an in-solution digestion protocol. Before MS-MS characterization, the peptide mixture was separated by capillary zone electrophoresis (CZE), the most commonly used separation mode in CE. In CZE, analytes migrate inside the capillary under the influence of an applied electrical field (14). In this study, with sheathless CE–ESI-MS-MS analysis it was possible to characterize the amino acid sequence from a single 200-fmol injection of a mAb tryptic digest. Simultaneously, glycosylation structures and relative abundances for various glycoforms were characterized. In addition, several types of PTMs of interests were identified and their relative abundances were estimated. This study using sheathless CE–ESI-MS-MS demonstrates the value of using an electrophoretic separation before MS-MS analysis, given that it was possible, in some cases, to separate very similar peptides that differed only by a single amino acid. The specificity provided by the CE separation mechanism and its suitability to the ESI process enriches the level of characterization that can be achieved from a single experiment and emphasizes the benefits of using CE–MS-MS for biologics characterization.
ExperimentalMAb Sample Preparation
The multilevel characterization of mAbs by sheathless CE–ESI-MS-MS is based on an adapted bottom-up proteomics experiment. In this approach, mAb samples first undergo tryptic digestion using an in-solution protocol. To maximize digestion yield, trastuzumab samples are first denatured using the RapiGest surfactant (Waters). After digestion, the surfactant is cleaved and forms a precipitate by lowering the pH of the mixture, which can be easily discarded. After proteolytic digestion, no solid-phase extraction (SPE) sample cleanup should be performed, because it will often lead to the loss of the most hydrophilic peptides and is in general not required for CE–ESI-MS-MS experiments. The resulting peptide mixture is then diluted to the desired final concentration (2.2 µM) using ammonium acetate at pH 4.0.
CE–ESI-MS Hyphenation
When performing CE–MS experiments, the instrument interfaces must be carefully considered and handled. Several types of CE–MS interfaces are currently available, relying on different systems and exhibiting specific characteristics (15–18). For mAb primary structure characterization, our work was performed using a novel CE–ESI-MS coupling recently made commercially available (CESI8000, Sciex). This sheathless setup guarantees optimal sensitivity as a result of the low flow rate (<50 nL/min) generated during the separation and the narrow internal diameter (30 µm) (19). These two characteristics tremendously favor the ionization process, providing enhanced sensitivity and improving the MS data generated. Using hydrodynamic injection, a volume of 91 nL was injected into the bare fused-silica capillary (i.d. 30 µm, 92 cm) of the CE instrument. The background electrolyte used to perform the separation was composed of 10% acetic acid and separation was performed by applying a voltage of -20 kV. The CE instrument was coupled to a 5600+ TripleTOF hybrid mass spectrometer (Sciex), which is composed of several quadrupoles and a time-of-flight mass analyzer, making it possible to perform MS-MS experiments. CE–ESI-MS-MS experiments were recorded using a Top10 information-dependent acquisition approach, which given the acquisition rate of the MS instrument, provided a duty cycle of 1.79 s while maintaining a resolution greater than 40,000 in MS and 25,000 in MS-MS.
Regarding data treatment, peptide identification can be automated using conventional search algorithms such as Mascot or BiopharmaView, the latter of which is dedicated to this type of characterization. Nevertheless, complementary manual data treatment may be required to make the most out CE–ESI-MS-MS data, especially in cases of small tryptic peptides (<4 amino acids) or to characterize glycopeptides.
Results and DiscussionAmino Acid Sequence Characterization
Using the sheathless CE–ESI-MS system, tryptic peptide mixtures were separated in the CE capillary based on their charge state in solution as well as their hydrodynamic radius, as shown in Figure 1. Peptides were identified from MS-MS spectra in an approach similar to that used in bottom-up proteomics experiments.
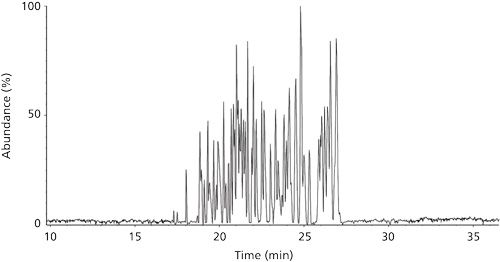
Sequence coverage of 100% of both the heavy chain and light chain was obtained from a single injection, as shown in Figure 2 (20). Despite the diversity of tryptic peptides generated from proteolytic digestion, complete sequence coverage was achieved through the identification of peptides without miscleavages or PTMs (21). The ability to completely characterize the amino acid sequence is mainly attributed to the properties of CE separation. Given the absence of stationary phase, peptide separation does not result from interactions with another medium; peptides migrate under the influence of the electrical field applied, regardless of their chemical nature, thus ensuring their successful transfer to the mass spectrometer. This advantage of CE is demonstrated by the identification in our experiments of a wide variety of peptides-including small peptides (≤ 3 amino acids) and a peptide composed of 63 amino acids (peptide HT15, position 151–213) and having a molecular mass greater than 6 kDa-under the same separation conditions.

In addition, the MS-MS spectra demonstrate a remarkable quantity of y and b ions, corresponding to the peptide backbone fragmentation. These ions are used for peptide identification in peptide fragment fingerprinting. Using this approach, peptide identifications are based on the mass measurements of both complete peptides and fragmentation products, thus increasing the confidence of the identification. Using CE–ESI-MS-MS data from a single experiment, it was possible to systematically identify more than 70% of the y and b ions composing the different mAbs studied; in the case of trastuzumab, this value was >90% (21). The capacity to generate and identify a large number of fragments is explained by the outstanding ionization efficiency provided by the sheathless CE–ESI-MS coupling. The reduced flow rate generated during separation and the narrow inner diameter of the capillary make it possible to produce droplets with a reduced diameter. Those small droplets are favorable to the ESI process and therefore ionization efficiency is improved. Improved ionization efficiency improves the quality of the MS-MS spectra, making it possible to obtain more fragment ions, as a result of the presence of an increased number of ions in the collision cell, and this improvement can be seen on the latest generation of mass spectrometers. Fragmentation spectra then provide important information regarding the exact amino acid sequence. For the characterization of trastuzumab, it was possible to fully map the amino acid sequence on the variable regions of the mAb. The variable regions of mAbs contain the complementarity-determining regions (CDR) and as a consequence are closely involved in antigen recognition. The ability to map the amino acid sequence of such a sensitive part of a biologic, with a high degree of accuracy, is a huge asset for R&D and reinforces the value of performing analytical characterization by CE–ESI-MS-MS.
Our studies, using technical replicates as well as several different monoclonal antibodies, have shown that this level of characterization of the amino acid sequence using sheathless CE–ESI-MS-MS is robust (21). In addition, another group recently described similar results using CE–ESI-MS-MS (22). Given these capabilities, sheathless CE–ESI-MS-MS appears to be an efficient and valuable technique for this type of application, providing, in a single analysis, a large amount of information about the amino acid sequence of a protein, and making it possible to identify amino acid substitution or potential transcription mismatches, in broader fields of study than the characterization of protein therapeutics (23).
Glycosylation Structural Characterization and Abundance Estimation
The glycosylation of a biologic is considered a critical quality attribute (CQA) and is subject to intense scrutiny by both biopharmaceutical companies and regulatory agencies, and can be a major source of batch-to-batch heterogeneity in biologics production (24). For instance, glycosylation can influence stability or solubility. In the case of mAbs, glycosylation affects the quaternary structure of the protein, which in turn affects antibody-dependent cell-mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) (25).
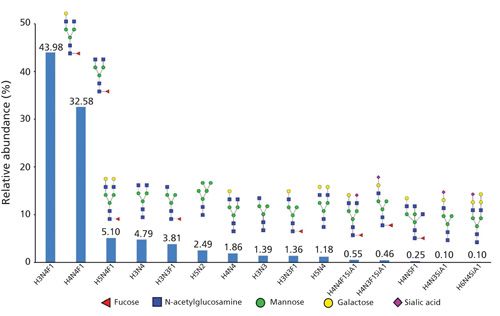
CE–ESI-MS-MS data previously used for the characterization of the amino acid sequence can also be used to locate and characterize the structure of glycoforms expressed by a biotherapeutic protein. In our study, collision-induced dissociation (CID) MS-MS spectra showed the fragmentation of the glycan moiety, making it possible to deduce the carbohydrate composition (Figure 3). Once again, the excellent quality of MS-MS spectra provided a large number of fragment ions, and in most cases this made it possible to unambiguously characterize the structure and carbohydrate composition of the glycosylation. To complement the glycoprofiling performed by sheathless CE–ESI-MS-MS, the MS signal was used to estimate the relative abundance of each glycoform. Those results are shown in Figure 4. The results obtained demonstrate the ability of this analytical approach to simultaneously characterize a significant number of glycoforms; for trastuzumab, 15 different glycoforms were characterized simultaneously.
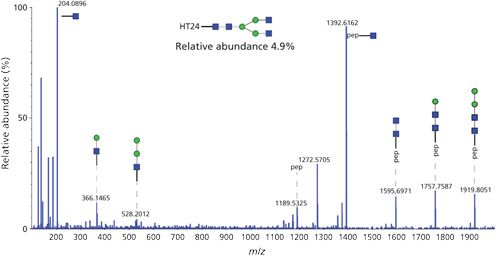
Glycosylation characterization was performed without prior glycan release, as it is commonly performed in similar studies (26). Because of the presence of the glycan still bonded to the peptide, glycopeptides tend to be significantly hydrophilic. Therefore, they may not be sufficiently retained on reversed-phase stationary phases and may be difficult to characterize among the other peptides, motivating the implementation of specific analytical methods (27). Because separation in CE is driven only by the electrical field applied to the capillary without interaction with a stationary phase, glycopeptides are separated regardless of their hydrophobicity, which reveals the advantages of CE separation with this particular type of peptide as they have been shown by different groups (28,29).
Because characterization with CE–ESI-MS-MS is performed on the peptide level, it is possible to precisely locate the amino acid bearing a glycosylation. Moreover, when multiple glycosylation sites are present on the protein, as is often the case, it is possible to consider these sites independently, for structural characterization as well as relative abundance estimation. Relative quantification makes it possible to characterize major glycoforms like G0F or G1F, as well as low-abundant glycoforms, including some exhibiting sialic acids; this demonstrates that the dynamic range of sheathless CE–ESI-MS-MS is sufficient for in-depth glycosylation characterization (21).
Just as with amino acid sequence characterization, the ionization efficiency provided by the sheathless CE–ESI-MS coupling system gives it optimal sensitivity for glycopeptide identification. However, CE separation also makes it possible to obtain partial separation of the glycopeptides generated from tryptic digestion. For example, two glycopeptides having a difference of only one galactose can be completely separated by CE, while a difference of one fucose led to the partial separation of the peptides in question. In cases of comigration, those different glycopeptides would most likely compete against each other during the ESI ionization process, thus lowering the sensitivity of the signal, especially for the low-abundant glycopeptides. Glycoprofiling data obtained using sheathless CE–ESI-MS-MS have been demonstrated to be comparable between different samples, which can be useful in the context of a biosimilarity assessment (23).
PTM Hotspot Identification
Because they are proteins, biotherapeutics are subject to various PTMs, such as oxidation, deamidation, and acetylation. Such PTMs can be induced by an uncontrolled production process or during long-term sample storage. In the biopharmaceutical industry, a few of these modifications are referred as PTM hotspots. Those hotspots, specific to each biotherapeutic protein, are described in the literature as influencing the affinity between a mAb and its corresponding antigen, and thus such a PTM can, in some cases, reduce the therapeutic molecule’s potency. Therefore, PTM hotspots can be considered a CQA and may be used to monitor potential degradations of the sample in long-term stability studies. CE–ESI-MS-MS data, previously used for amino acid sequence and glycosylation characterization, has also been used to identify PTM hotspots in trastuzumab. Indeed, CE–ESI-MS-MS data showed the particular benefit of using the electrokinetically driven separation of CE to identify these PTMs.
The first PTM of interest studied was N-terminal glutamic acid cyclization. Sheathless CE–ESI-MS-MS experiments demonstrated that the modified peptide could be separated from the intact version of the same peptide in several minutes. The selectivity of CE for the N-terminal glutamic acid cyclization occurs because this modification creates an additional charge to the modified peptide in solution as a result of the pH used to perform the separation (30). As a consequence, these peptides exhibited significantly different mobilities leading to their complete separation. In addition, MS-MS fragment annotation made it possible to unambiguously characterize those two different peptides (20).
Oxidation of methionine was also studied. For some specific sites, especially in the constant domain of the mAb, methionine oxidation sites are also considered hotspots. This modification entails a mass increase of 15.99 Da for the modified peptide compared to the unmodified one, but the charge of the peptide is not modified. For this type of PTM, the selectivity provided by the CE separation mechanism enabled separation of the oxidized peptide from its unmodified equivalent. In addition, the excellent MS-MS spectral quality made it possible to precisely characterize the amino acid bearing the modification, even in the case of tryptic peptides composed of several methionines (21).
Deamidation is a common PTM occurring on asparagine residues, and involves a mass difference of 0.98 Da between the modified and unmodified peptide (31). Electropherograms recorded during sheathless CE–ESI-MS-MS experiments demonstrated complete separation of a deamidated peptide from the same peptide without the modification. And as was seen with the analysis of other types of PTMs, the amino acids affected by the modification were unambiguously identified using the MS-MS spectra, as shown in Figure 5. The ability to separate peptides showing such subtle differences is valuable in the context of the primary structure characterization. Without separation before their transfer to the ESI source, those peptides would most likely compete during the ionization process, thus lowering the sensitivity of the MS signal, which might prevent successful characterization of the modification. Similarly, if the peptide happened to be doubly charged, its isotopic profiles would overlap, preventing convenient MS selection and MS-MS fragmentation, making characterization problematic. To complete the discussion of deamidation characterization, we should note that estimation of the occurrence levels was performed using the MS signal for several hotspots. Results demonstrated that the digestion protocol induced a reduced level of deamidation, which can be significant in the case of uncontrolled tryptic digestion (23).
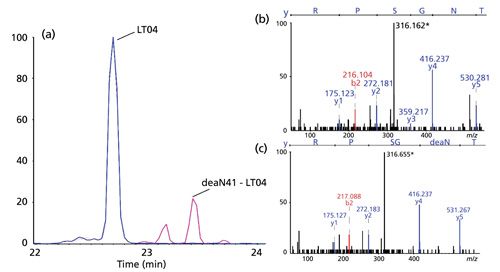
Finally, aspartic acid isomerization was considered. This type of PTM is particularly relevant, because it does not involve a mass difference between the modified and unmodified peptides, and therefore it cannot be identified, considering the type of mass spectrometer used for this work, directly from the MS-MS data (Figure 6), without applying specific analytical methods (31). Sheathless CE–ESI-MS-MS results proved that the conditions used during the separation made it possible to completely separate digested peptides having the same amino acid composition but different isomers of aspartic acid, as can be seen in Figure 6. Because MS-MS spectra of both peptides were similar, as expected, further studies were performed using synthetic peptides to prove the selectivity of CE separation for aspartic acid isomerization. The ability to separate peptides composed of different isomers of aspartic acid is ascribed to a change in the hydrodynamic radius of the peptide depending on the aspartic acid isomer, which during the electrophoretic separation creates a significant difference in the mobilities of the peptides in question.
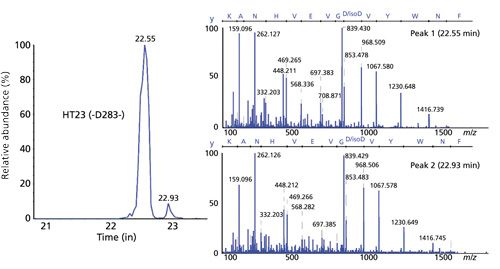
As with the analysis of the other PTMs discussed above, every peptide potentially exhibiting aspartic acid isomerization hotspots was successfully identified simultaneously using the CE–ESI-MS-MS data, and therefore the abundance levels of these PTMs also could be estimated using the MS signal. In this particular example, the implementation of an electrophoretic separation before MS analysis made it possible to increase the amount of information about the primary structure of trastuzumab that could be gained from a single analysis, because without the electrophoretic separation, the modified peptide would not have been identified from the MS data only. And just as with the analysis of asparagine deamidation, the separation of the modified peptide guarantees optimal ionization of both peptides by preventing ionization competition and therefore maximizing the sensitivity of the MS data generated. The benefit of this capability is seen in the ability to detect weak modification levels (< 2%) of trastuzumab using the MS signal for aspartic acid isomerization as well as asparagine deamidation (23).
Conclusions
Recently, our group developed an innovative analytical methodology for the characterization of the primary structure of biotherapeutic proteins using sheathless CE–ESI-MS-MS instrumentation. Using a single injection of a trastuzumab peptide mixture corresponding to a quantity of 200 fmol, it was possible to achieve amino acid sequence characterization with 100% sequence coverage. For the first time, complete sequence coverage could be achieved using a bottom-up proteomic approach from a single analysis of a tryptic digest. The sheathless CE–ESI-MS interfacing system was shown to favor the ESI process, as a result of the design of the interface and the background electrolyte employed. The outstanding sensitivity provided by the system has been shown to improve MS-MS spectral quality, and it was possible to systematically recover more than 90% of the b and y ions of trastuzumab, giving valuable information regarding the amino acid sequence, for example. Using data from the same analysis, glycosylation was also analyzed. We showed that it was possible, using the developed method, to characterize the structure and position of 15 glycoforms expressed by trastuzumab while estimating the relative abundance of each glycoform in a consistent manner. Using the very same CE–ESI-MS-MS data, various PTM hotspots were characterized and their occurrence levels estimated. In the characterization of PTMs, including glycosylation, another aspect of the implementation of an electrophoretic separation before the MS analysis was seen. Indeed, our study of CE separation selectivity demonstrated the ability to separate peptides having only minor differences, such as an asparagine deamidation or being composed of different isomers of aspartic acid. The capacity to separate a modified peptide from its intact homologue before transfer to the ionization source enables precise identification of the modified amino acid by suppressing ionization competition, therefore explaining the excellent sensitivity that could be achieved for estimates of the abundance of a given PTMs. The CE–ESI-MS-MS method was shown to provide, in a reduced analysis time, a large set of information about the primary structure of a biotherapeutic. Considering the characterization required from the regulatory agencies for the market introduction of a new biologic or a biosimilar, sheathless CE–ESI-MS-MS possesses many attractive properties and enables comprehensive and robust characterization. Developments achieved by our group show that sheathless CE–ESI-MS-MS represents an innovative analytical tool that complements current techniques for biopharmaceutical R&D.
Acknowledgments
The authors would like to thank Sciex for lending us a CESI prototype instrument, and E. Betgovargez and Dr. M. Anselme from Sciex for their support. The authors would like also to express their gratitude to Dr. E. Wagner-Rousset, M.C. Janin-Bussat, and O. Colas of the Centre d’Immunologie Pierre Fabre, in St. Julien en Genevois, France for helpful discussions about antibody structural characterization.
References
(1) A. Beck, P.J. Carter, H.P. Gerber, A.A. Lugovskoy, T. Wurch, J.R. Junutula, R. Kontermann, R. Mabry, and T. Ghayur, mAbs5, 339–357 (2013).
(2) J.M. Reichert, mAbs6, 799–802 (2014).
(3) M. Hay, D.W. Thomas, J.L. Craighead, C. Economides, and J. Rosenthal, Nat. Biotechnol.32, 40–51 (2014).
(4) A. Beck, S. Sanglier-Cianférani, and A. Van Dorsselaer, Anal. Chem.84, 4637–4646 (2012).
(5) A. Beck anad J.M. Reichert, mAbs5, 621–623 (2013).
(6) S. Rosati, N.J. Thompson, and A.J.R. Heck, Trends Anal. Chem.48, 72–80 (2013).
(7) L. Fornelli, D. Ayoub, K. Aizikov, A. Beck, and Y.O. Tsybin, Anal. Chem.86, 3005–3012 (2014).
(8) M. Biacchi, R. Bhajun, N. Saïd, A. Beck, Y.N. François, and E. Leize-Wagner, Electrophoresis35, 2986–2995 (2014).
(9) A. Beck, E. Wagner-Rousset, D. Ayoub, A. Van Dorsselaer, and S. Sanglier-Cianferani, Anal. Chem.85, 715–736 (2013).
(10) A. Beck, H. Diemer, D. Ayoub, F. Debaene, E. Wagner-Rousset, C. Carapito, A. Van Dorsselaer, and S. Sanglier-Cianferani, Trends Anal. Chem. 48, 81–95 (2013).
(11) J.A. Olivares, N.T. Nguyen, C.R. Yonker, and R.D. Smith, Anal. Chem. 59, 1230–1232 (1987).
(12) M. Moini, Anal. Chem.79, 4241–4246 (2007).
(13) J.M. Busnel, B. Schoenmaker, R. Ramautar, A. Carrasco-Pancorbo, C. Ratnayake, J.S. Feitelson, J.D. Chapman, A.M. Deelder, and O.A. Mayboroda, Anal. Chem.82, 9476–9483 (2010).
(14) J. Jorgenson, K. Lukacs, Science222, 266–272 (1983).
(15) G. Bonvin, J. Schappler, S. Rudaz, J. Chromatogr. A1267, 17–31 (2012).
(16) P. Lindenburg, R. Haselberg, G. Rozing, and R. Ramautar, Chromatographia78, 367–377 (2015).
(17) A.A.M. Heemskerk, A.M. Deelder, and O.A. Mayboroda, Mass Spectrom. Rev.9999, 1–13 (2014).
(18) E.A. Redman, N.G. Batz, J.S. Mellors, and J.M. Ramsey, Anal. Chem.87, 2264–2272 (2015).
(19) R. Gahoual, J.M. Busnel, P. Wolff, Y.N. François, and E. Leize-Wagner, Anal. Bioanal. Chem.406, 1029–1038 (2014).
(20) R. Gahoual, A. Burr, J.M. Busnel, L. Kuhn, P. Hammann, A. Beck, Y.N. François, and E. Leize-Wagner, mAbs5, 479–490 (2013).
(21) R. Gahoual, J.M. Busnel, A. Beck, Y.N. François, and E. Leize-Wagner, Anal. Chem.86, 9074–9081 (2014).
(22) C. Lew, J.L. Gallegos-Perez, B. Fonslow, M. Lies, and A. Guttman, J. Chromatogr. Sci.53, 443–449 (2015).
(23) R. Gahoual, M. Biacchi, J. Chicher, L. Kuhn, P. Hammann, A. Beck, E. Leize-Wagner, and Y.N. François, mAbs6, 1464–1473 (2014).
(24) C.N. Damen, W. Chen, A. Chakraborty, M. Oosterhout, J. Mazzeo, J. Gebler, J.M. Schellens, H. Rosing, and J. Beijnen, J. Am. Soc. Mass Spectrom.20, 2021–2033 (2009).
(25) D. Ayoub, W. Jabs, A. Resemann, W. Evers, C. Evans, L. Main, C. Baessmann, E. Wagner-Rousset, D. Suckau, and A. Beck, mAbs5, 699–710 (2013).
(26) L.R. Ruhaak, C. Huhn, W.J. Waterreus, A.R. de Boer, C. Neusüss, C.H. Hokke, A.M. Deelder, and M. Wuhrer, Anal. Chem.80, 6119–6126 (2008).
(27) K. Stavenhagen, D. Kolarich, and M. Wuhrer, Chromatographia78, 307–320 (2015).
(28) A.A.M. Heemskerk, M. Wuhrer, J.M. Busnel, C.A.M. Koeleman, M.H.J. Selman, G. Vidarsson, R. Kapur, B. Schoenmaker, R.J.E. Derks, A.M. Deelder, and O.A. Mayboroda, Electrophoresis34, 383–387 (2013).
(29) R.G. Jayo, M. Thaysen-Andersen, P.W. Lindenburg, R. Haselberg, T. Hankemeier, R. Ramautar, and D.D.Y. Chen, Anal. Chem.86, 6479–6486 (2014).
(30) D. Chelius, K. Jing, A. Lueras, D.S. Rehder, T.M. Dillon, A. Vizel, R.S. Rajan, T. Li, M.J. Treuheit, and P.V. Bondarenko, Anal. Chem.78, 2370–2376 (2006).
(31) H. Yang and R.A. Zubarev, Electrophoresis31, 1764–1772 (2010).
Rabah Gahoual, Michaël Biacchi, Yannis-Nicolas François, and Emmanuelle Leize-Wagner are all with the Laboratoire de Spectrométrie de Masse des Interactions et des Systèmes (LSMIS), at the University of Strasbourg, in Strasbourg, France. Rabah Gahoual is also with he Laboratory of Bioanalytical Chemistry and Spectroscopy at the Free University (Vrije Universiteit) in Amsterdam, the Netherlands. Alain Beck is with the Centre d’Immunologie Pierre Fabre, in Saint-Julien-en-Genevois, France. Jean-Marc Busnel is with Beckman Coulter Inc., in Marseille, France. Direct correspondence to: yfrancois@unistra.fr.

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.











