Column Highlights of HPLC 2007
LCGC North America
Columnist Ron Majors covers some of the highlights of HPLC 2007 including honorary sessions, best poster awards, and the latest developments in HPLC column technology.
The 31st International Symposium on High Performance Liquid Phase Separations and Related Techniques, which alternates between Europe and North America, was held for the first time in Belgium at the International Convention Center, Ghent, Belgium June 17–21, 2007. More affectionately known as HPLC 2007, the symposium is the premier scientific event for bringing together the myriad techniques related to separations in liquid and supercritical fluid media. Cochaired by Professors Patrick Sandra of Ghent University (Ghent, Belgium) and Jacques Crommen, University of Liege (Liege, Belgium), the symposium assembled just under 1200 scientists from a total of 45 countries. This number did not include vendor representatives from over 70 exhibitors for the three-day instrument, software, and consumables exhibition.

Ronald E. Majors
The five-day-plus event had a total of 122 oral presentations, many presented during simultaneous sessions, 800 posters (the largest for this meeting to date) in sessions with 28 themes. With an ample social event schedule, 17 vendor workshops, eight tutorial educational sessions, and eight short courses held during the previous weekend, attendees had their hands full deciding how to allocate their time. The tutorials were particularly well attended covering topics such as chemometrics, particulate columns versus monoliths, theory, electrodriven separations, cleanup of biofluids for liquid chromatography (LC)–mass spectrometry (MS), enantiometric separations, and polymer separations.
Obviously, HPLC was the predominant technology in the technical sessions at the symposium. From a perusal of the poster and oral presentation abstracts, I broke down some of the predominant areas of coverage in this year's symposium. These tables are useful to spot trends in the technology and applications of the predominant liquid phase researchers in the world. Many of the topics that are currently "hot" in the separations sciences were introduced in this series.

Table I provides a rough breakdown of the coverage of liquid phase technology and techniques in the separation sciences. Compared with HPLC 2006 (1), some definite shifts in technology emphasis were noted. Although column technology always leads the pack, this year, half of the column papers dealt with silica and polymeric monoliths, about equally divided in their emphasis. Sample preparation topics, always in the top three categories of technology, were a clear number two with solid-phase extraction (SPE), selective phases such as molecularly imprinted polymers, restricted access media, and various forms of liquid–liquid extraction getting attention. The third biggest technology topic was ultrahigh-pressure LC (UHPLC) and high-throughput HPLC. There seemed to be some confusion among chromatographers trying to associate the technique with the nomenclature. Most of the papers talking about these techniques were using smaller particles (sub-2-μm in diameter) packed into short columns (for example, lengths of 20–50 mm) for the purposes of running high-speed separations. For this reason, I moved the sub-2-μm papers into this classification and the percentage of talks classified under columns has come down from last year's total (1) because of this shift. Surprisingly, even with a number of high-pressure pumps and systems coming onto the market, very few papers at HPLC 2007 reported applications at pressures over 400 bar. Other areas that had great interest were electrophoretic techniques — for example, capillary electrophoresis (CE), capillary zone electrophoresis (CZE), micellar electrokinetic chromatography (MEKC) and isoelectric focusing (IEF) — high-temperature separations, and multidimensional separations. The biggest losers compared to last year were lab-on-a-chip and microfluidic studies, which seemed to evaporate overnight. Perhaps this dropoff is more reflective of the location of the meeting, which tends to attract a larger number of Europeans when held in America where the miniaturization movement is stronger.
Table II is a breakdown of the most popular application areas. Again, the separation of proteins and peptides was the leading area of applications this year. The biggest growth was in the analysis of foods and beverages. Food safety has become an important area of focus, especially in view of the many examples of food contamination that have made headlines in the past year.
I also tabulated the detection principles (Table III) that were used. Not every abstract indicated the detector used, so only those who provided this information were counted. The category assignments were based upon the main emphasis of a particular scientific paper as well as separation and detection techniques used. MS clearly dominates the detection category with UV detection a distant second. If one adds up the use of MS in chromatography and electrophoretic techniques, one half of the papers presented at HPLC 2007 used this detection technique. There was even more growth in the tandem MS category, which allows more definitive assignment of molecular structural information.
In this installment of "Column Watch," I will present some of the scientific highlights of HPLC 2007. This report will emphasize the various awards and honorary sessions taking place, the scope of the plenary lectures, column and phase developments, temperature studies, multidimensional chromatography, detection highlights, and a few significant applications trends. I will report new sample preparation technologies in a future "Sample Preparation Perspectives" column. Because it was virtually impossible for one person to cover all oral presentations and posters adequately, my coverage will somewhat reflect a personal bias, although I was able to get summaries from some of my colleagues who are acknowledged at the end of this column.
Horvath Award
For the second year in a row, the Horvath Award sessions, named for the late Prof. Csaba Horvath, one of the founders of this series and a mentor of young scientists, were featured. This award — supported by HPLC Inc., a nonprofit group under the guidance of the Permanent Scientific Committee — was established for young scientists in the separation sciences under the age of 35. The award, based upon the best oral lecture presented in the Horvath Sessions, was selected by a jury from the Permanent Scientific Committee and consists of a travel grant to and free registration for HPLC 2008 as well as a trophy. This year there were 10 nominees and half were female scientists. This year's winner was Caterina Temporini from the Department of Pharmaceutical Chemistry, University of Pavia (Pavia, Italy). Her coauthors were E. Calleri, L. Dolcini, C. Sasso, F. Corana, G. Massolini, and G. Gaccialanza, all from the University of Pavia. The title of her award winning presentation was "Integrated LC/MSn Analytical Strategies for Post-Translational Modifications in Protein Analysis." The researchers investigated two model glycoproteins that were digested rapidly online using a new pronase-MER. Coupling the bioreactor to a carbon-based trap column allowed selective enrichment of the glycopeptides before the normal phase LC step. They also studied phosphoproteins using enrichment with TiO2 and followed the digested phosphopeptides by reversed-phase HPLC. A photo of the 2007 Horvath Award winner and presenter Dr. John Frenz, Chairman of HPLC 2006 and a member of the Permanent Scientific Committee, is shown in Figure 1.

Figure 1: John Frenz, member of the Permanent Scientific Committee, presented Caterina Temporini of the Unversity of Pavia, Pavia, Italy with the 2007 Horvath Award.
Poster Sessions and Best Poster Awards
The mainstay of HPLC 2007 was the poster sessions where more detailed applications and methodology studies were reported, often in very specific areas, and face-to-face discussions with the authors were conducted. Fortunately, many of the poster authors were kind enough to provide small reproductions of their poster papers that could be taken for later perusal. Some collected business cards and addresses for sending poster reprints by mail or e-mail. With this year's overwhelming number of posters and limited poster board space, the posters were split into two two-day sessions of roughly 420 and 380 each, making it quite difficult for attendees to cover all the posters. Because the days that the posters were staffed by presenters were staggered, the sessions did not appear to be crowded.
This year I once again had the privilege of leading the Poster Committee that chooses the top three posters at the symposium. With over 800 posters to cover, poster review was a challenging task. Fortunately, I had the able assistance of 45 members of the Poster Committee who devoted a great deal of time and worked very hard to narrow down the huge collection of posters and helped to select the three winners by the end of the fourth day of the symposium. I want to individually recognize them in by providing their names and affiliations in Table IV. Overall, the committee felt that the quality of posters was again outstanding and showed continued improvement over previous symposia in this series.
The Best Poster Awards, sponsored by Agilent Technologies (Wilmington, Delaware), were announced at the Closing Session. The three winners received Amazon.com gift checks. This year's winning poster was titled "Novel Monolithic Poly(p-methylstyrene-co-bis(p-vinylbenzyl)dimethylsilane) Capillary Columns for Biopolymer Separation" by authors Wolfgang Wieder, L. Trojer, C. Bisjak, and G. Bonn of the Institute of Analytical Chemistry and Radiochemistry, University of Innsbruck (Innsbruck, Austria) and S. Lubbad Department of Chemistry, Alazhar University-Gaza (Gaza, Palestinian Territory, Occupied). In their paper, the authors showed how they were able to synthesize hydrophobic organo-silane monoliths in 200-μm i.d. fused-silica capillaries by thermally initiated free radical polymerization reactions. By investigating various polymerization parameters such as the monomer-to-porogen ratio, they were able to tune their monolith to be optimal for the separation of various biopolymers (for example, proteins, peptides, and oligonucleotides). The monolith displayed very low swelling characteristics in organic solvents. Figure 2 shows a photograph of graduate student Wolfgang Wieder receiving his prize at the Closing Session of HPLC 2007.

Figure 2: Wolfgang Weider of the University of Innsbruck, Austria received the Best Poster Award presented by Poster Chairman Ron Majors.
Winners of the second best poster, entitled "High Performance Reversed-Phase Separations Using Micro-Fabricated Columns with Perfectly Ordered Support Structures," were Hamed Eghbali, W. De Malsche, D. Clicq, J. Vangelooven, and G.Desmet from the Vrije Universiteit Brussel (Brussel, Belgium), and H. Gardeniers of MESA+ Research Institute (Enschede, Netherlands). Their poster covered work on the use of micromachining to produce perfectly ordered chromatographic supports that provide dramatic increases in column efficiency with a decreased flow resistance. They performed reversed-phase separations in an array of nonporous silicon pillars with a diameter of approximately 4.3 μm and an external porosity of 55%; a three-component mixture could be separated in 3 s with 4000–5000 plates realized over a column length of 10 mm. Of particular note is that this research group has finished in the top three posters awards in three out of the last four years. Figure 3 provides a photo of Hamed Eghbali receiving his Award for the second prize.

Figure 3: Hamed Eghbali of the Vrije Universiteit Brussel, Belgium receives his second place Best Poster Award.
The award for the third best poster, titled "Comprehensive Normal Phase × Reversed Phase Liquid Chromatography (NPLC×RPLC) with Parallel Second Dimension Columns," was presented to Isabelle François of the University of Ghent, Belgium, along with coauthors A. de Villiers of the University of Stellenbosch (Stellenbosch, South Africa) and B. Tienpont and P. Sandra of Research Institute for Chromatography (Kortrijk, Belgium). The linking of two orthogonal separation modes via a two-position, 10-port switching valve with two storage loops, the latter having a volume equivalent to the mobile phase quantity from the first dimension, allowed the workers to add a second column in the second dimension, which increased overall peak capacity and chromatographic resolution. An effective peak capacity of 1095 was achieved for a lemon oil extract, more than two times the peak capacity established with a conventional loop interface. Figure 4 shows Isabelle François receiving her award.

Figure 4: Isabelle François of the University of Ghent, Belgium collects her third place Best Poster Award.
At HPLC 2007, the Pfizer Analytical Research Center (Parc) presented awards for the best contributions (oral and poster) related to innovations in pharmaceutical analysis. Unfortunately, I do not have the list of the winners to report here.
Honorary Sessions: Verzele and Unger
Two of the pioneers in chromatography were honored at HPLC 2007: Prof. Maurits Verzele of Ghent University and Prof. Klaus Unger of the Johannes Gutenberg Universitat-Mainz (Mainz, Germany). Both officially retired from their universities, Unger in 2001 and Verzele in the late 1980s. Both of these educators have graduated many Ph.D. degrees, have hundreds of publications, have published textbooks, filed for patents, have organized symposia, have won many awards, and have been a friend to many retired and active chromatographers.
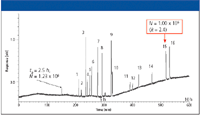
Figure 5: The first million plate chromatogram on a silica-based monolith column separation of PAHs (15). Column: monolithic silica C18, effective length of 1238 cm; mobile phase: 80% acetonitrile; detection: UV absorbance at 254 nm; temperature: 30 °C. Peaks: 1 = naphthalene, 2 = acenaphthylene, 3 = fluorene, 4 = acenaphthene, 5 = phenanthrene, 6 = anthracene, 7 = fluoranthene, 8 = pyrene, 9 = chrysene, 10 = benz(a)anthracene, 11 = benzo(b)fluoranthene, 12 = benzo(k)fluoranthene, 13 = benzo(a)pyrene, 14 = dibenz(a,h)anthracene, 15 = indeno(1,2,3-cd)pyrene, 16 5 benzo(g,h,i)perylene.
Prof. Verzele, of the Organic Chemistry Department, was an early pioneer in gas chromatography (GC), particularly in the area of preparative GC and in the coating of capillary columns. He also did early studies in GC–MS, temperature programming optimization and packed column supercritical fluid chromatography (SFC). Many of his papers were coauthored with Prof. Sandra, cochairman of HPLC 2007, and now professor at the same institute. In the HPLC area, Verzele's contributions ranged from micro- and capillary LC to phase synthesis, column packing studies, and preparative LC. He was one of the early workers who observed the improvements in column efficiency using small microparticulates in the 2-μm range. Chris DeWaele, now of Bio-Rad RSL (Nazareth, Belgium), coauthored many HPLC papers with Prof. Verzele.
Maurits is an expert on the chemistry of beer making and his studies on hop bitter acids are world renowned. In addition, his work in the area of pepper aroma and the characterization of pepper extracts are equally well known. He was noted for his practical approaches in solving "real world" chromatography problems.
Prof. Klaus Unger was also one of the early researchers in HPLC column technology. He first synthesized spherical porous silica gel microparticulates in 1973, describing an emulsification and polycondensation process that is still in use today. Two decades later, Unger was still working in this area, having published papers on sub-2-μm packing, now a "hot" topic in HPLC. In 1979, he wrote a definitive book on porous silica, its properties, and use as support in column liquid chromatography, and it is used by many columns' specialists as their textbook today. In the 1980s, Klaus and his research group became more involved in the life science applications of chromatography, and he was one of the founders of the International Symposium on the Separation of Proteins, Peptides and Polynucleotides (ISPPP) that is still going strong today. In the 1990s, his interests turned to multidimensional chromatography, capillary electrochromatography, and restricted access media (RAM), and he was a strong advocate of the EU Project on Reference Materials for HPLC. Although retired, Prof. Unger still carries on active research projects in proteomics and preparative LC.
New Column Technology Highlights
As shown in Table I and similar to the last seven years of HPLC Symposia coverage (1–7), the development and study of columns and stationary phases still dominates new technologies. At least seven oral sessions and an equivalent number of poster sessions were devoted to column technology, retention mechanisms, high-throughput applications, UHPLC, and the like. If one combines all papers pertaining to column technology, about 25% of the presentations at HPLC 2007 covered this topic. Despite all the advances made in column technology to date, investigations on further developments in academia as well as the commercial side are still taking place.
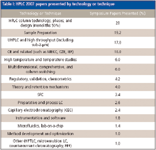
Table I: HPLC 2007 papers presented by technology or technique
From what I observed at HPLC 2007, a current "hot topic" in HPLC column technology was the discussion of the alternative approaches for developing faster separations and generating more column efficiency at lower pressure drop and which approach will be favored in the long run. These approaches are
- Packed columns with small porous particles (sub-2-μm)
- Packed columns with small porous particles (2–3 μm)
- Monoliths (silica and polymeric)
- Superficially porous packings (~2.7 μm)
In general, all these approaches work in balancing column efficiency, phase ratios, and column permeability. In the monolith approach, at least in the academic setting, improvements have been made in the homogeneity of the continuous beds and improvements in efficiency by adjusting the relative sizes of the macro- and mesopores, which can be varied independently using the sol-gel synthesis process, and has led to better overall chromatographic columns. By varying this ratio, monolith permeability can be affected, sometimes in a detrimental way, taking away one of the advantages of monoliths over packed beds.

Table II: Papers presented by application area
The sub-2-μm packed column approach uses conventional spherical silica particles but with sub-2-μm particles sizes being favored, each having various particle size distributions. Short columns, usually less than 50 mm in length, are run at high linear velocities, giving sample high throughput. On the other hand, if more theoretical plates are required, these small particles are packed into longer columns (up to 15 cm) but when these columns are run at higher flow velocities, more back pressure is generated. This new nomenclature of UHPLC has arisen to describe the higher back-pressure requirement. In addition, high temperatures are now fashionable to lower back pressure as well as improved solute mass transfer.
A number of "camps." mostly commercial entities, endorse particle sizes that are 2–3 μm in diameter. Because the particle diameter is larger than the sub-2-μm particles, the pressure drop is lower but efficiency is better than the more popular 3–3.5 μm particles. The arguments here revolve around the entire separation cycle time: higher temperatures are used to improve efficiency and lower back pressure; improved liquid chromatograph hydraulics are used to lower band dispersion and gradient reequilibration time; faster autosamplers, faster detectors, and faster data systems are used to decrease instrumental contributions to the cycle time.
The superficially porous particle (~2.7 μm) approach, a subset of the 2–3 μm particles in terms of pressure, has a solid inner core and a porous outer core. The outer core is sufficiently thin to allow rapid mass transfer into and out of the stationary phase; because the inner core is solid, analytes cannot penetrate any further. This diffusion path length is shorter than the porous particles of approximately the same diameter and roughly equivalent to the sub-2-μm particles. Proponents here say that you get the lower pressure drop of the larger particle and the efficiency of the smaller particle. Nothing was mentioned about the reduced amount of available stationary phase that determines sample capacity for larger mass injections.
Table III: Final committee
Monolith column technology: At HPLC 2007, just as capillary electrochromatography (CEC) did in the late 1990s to early 2000s, monolithic phases dominated columns' technology and applications' papers with around 50% of column-oriented presentations devoted to this technology.
Monolith columns have been desirable because they exhibit high permeability and low pressure drop (due to increased bed porosity), show good separation efficiency, have the absence of frits to confine the packing material, are easy to fabricate, and can be made fairly reproducibly. Although this technology has been around for several years, as a routine tool it has yet to see widespread acceptance, but with the continued academic advances reported at HPLC 2007, if commercialized, users should take more advantage of these columns. Reports were devoted to improving both polymer- and silica-based monoliths. Overall observations indicate that silica-based monoliths seem to work best for small molecules, while the polymeric monoliths are best for macromolecules. Both theoretical and practical studies have been applied to understanding how to increase phase ratios and homogeneity of the co-continuous structures of silica skeletons.
At HPLC 2007, K. Cabrera of Merck KGaA, Darmstadt, Germany presented an overview of the company's commercial silica monolith columns, pointing out the advantages (but none of the disadvantages). The composition of the monolith is governed by the tetramethyl orthosilicate (TMOS) to polyethyleneoxide (PEO) content. Because the quality of the monolith columns is determined by the homogeneity of the porous silica structure, based upon academic results reported last year at HPLC 2006, the company is now investigating differences in domain size by variation of the macropore and skeleton sizes. They are investigating the radial heterogeneity of the monoliths by using the Knox infinite diameter effect by injecting at different spots on the top of the column and looking at elution profiles for inconsistencies. The axial homogeneity can be determined by cutting the monolith into various lengths. In the process of improving their processes, they were able to construct a 1-m column that generated over 200,000 plates in 40 min with a peak capacity of 436.
Major practical advances in silica-based monolith technology have occurred in the studies of Tanaka and coworkers from the Kyoto Institute of Technology (Japan). T. Ikegami of this laboratory presented their latest findings on silica-based monoliths. This year, their work was devoted to increasing the plate count by constructing long monolithic columns, incorporating new chemistries into the backbone for selectivity changes, and using two-dimensional (2-D) separations to increase peak capacity. Single monolith columns of 2.5 m were operated at 3.83-mm/s linear velocity and generated approximately 200,000 theoretical plates at a pressure of 45 MPa. A monolithic silica column of 4.4 m was employed to separate isotopologues of benzene and toluene. By using the monodimensional HPLC, benzene and benzene-d1 were separated. A 1.3-m monolithic silica column was used to separate the 16 U.S. Environmental Protection Agency (USEPA) polyaromatic hydrocarbons (PAHs) in 72 min. Columns as long as 12.4 m were constructed by coupling shorter columns although in the process of connecting the columns some theoretical plates were lost. The 12.4-m monolithic silica capillary column provided 1,000,000 theoretical plates even at k = 2.4. The chromatogram showing this remarkable achievement is depicted in Figure 1.
Using in situ polymerization techniques, several different polar phases having attached amide, amine, quaternary ammonium, sulfonate, carboxyl, or phenyl groups were synthesized and used in the ion-exchange and hydrophilic interaction chromatography (HILIC) modes. The HILIC-type monolith columns were compared with comparable commercial particle packed columns and were found to be superior in terms of their lower H-u curves. In their comprehensive 2-D studies, using a conventional particle-packed column as the first dimension column and a very short (10–25 mm) monolith as the second dimension column, a peak capacity of 2750–3000 at 2.2–4 sigma modulation was demonstrated. A HILIC column coupled to a strong cation exchange monolith column set was used to demonstrate separations of peptides and proteins.
A poster paper also showed very long silica monolith columns. A group from Dublin City University and Centre for Bioanalytical Science, Dublin, Ireland, prepared 1-m long monoliths that generated 60,000 theoretical plates at MS compatible flow rates. In testing their column's performance, using gradient elution, peak capacities of 1200 were achieved in about 290 min.
H. Saito, from Nakanishi's group at Kyoto University, synthesized and studied monoliths prepared by the sol-gel process incorporating polyacrylic acid (HPAA) and PEO into the monolith. They induced phase separation parallel to the sol-gel transition and obtained gels with well-defined macroporous structure. They observed the formation of the monoliths with and without this phase separation using small-angle x-ray light scattering (SAXS), small-angle light scattering (SALS), and laser scanning confocal microscopy (LSCM) to verify homogeneity when varying the ratio (and the content) of HPAA or PEO. HPAA does not interact well with the siloxane being formed and stays in the solvent while PEO prefers the silxane phase. HPAA gives more homogeneous pores but the final product is not much different in terms of the final monolithic skeleton–porosity. These studies should give more insight into the preparation of an optimized monolith with the desired surface characteristics. Others reported the use of the Kyoto University approach to prepare hydrophilic as well as hydrophobic monoliths with varying degrees of porosity.
In addition to the award-winning poster cited earlier, in several other papers, silica monoliths were further modified by the incorporation of various functionalities into its backbone. One such modification involved the coating of silica capillary columns with poly(octadecylmethacrylate) to yield different selectivity compared with other reversed-phase silica monolith columns. Another consisted of an alkoxysilane phase containing a carboxyl functionality bonded to a monolith that had useful electroosmotic flow properties along with cation exchange properties in capillary electrochromatography. A number of poster papers prepared other cation ion-exchange monoliths by adsorbing cationic surfactants onto a silica surface; in some cases, the surfactant leached from the column with time, resulting in a change in retention. A better solution was the construction of stable ion-exchange phases based upon the adsorption of latex particles onto a polymer-based monolith. Anion-exchange monoliths could be prepared by a similar process. A diol phase was prepared by the reaction of a silica rod with gamma-glycidoxypropyltrimethoxysilane, and the resulting monolith was used for the separation of biomolecules.
Representing the polymeric monolith school, Frantisek Svec of the Molecular Foundry at the Lawrence Berkeley Laboratory (Berkeley, California) presented a talk on new tricks with old monoliths. In his presentation, he demonstrated the many possible uses of monoliths in the separation and sample preparation areas. One area of focus is the construction of polymeric monoliths in capillary columns and in the chip format. His group prepared methacrylate-based monoliths by thermally-initiated polymerization inside an LC chip format (Agilent Technologies, Santa Rosa, California). The polymeric monolith is able to fill the chip channel completely and can be used to separate proteins using a water–acetonitrile gradient over 5 min at a 4-μL/min flow rate. He went on to describe a GC column consisting of a polydivinylbenzene monolith inside of a 50 cm × 100 μm fused-silica capillary using temperature programming up to 300 °C, which he used to very rapidly separate a homologous series of volatile hydrocarbons up to C4. In the planar format, thin layer chromatography and matrix-assisted laser desorption ionization (MALDI) platforms can be constructed using macroporous monolithic structures without the need of additional matrix components. In sample preparation, monoliths have been applied to SPE. Svec also demonstrated the use of monoliths to construct an immobilized enzyme (pepsin) reactor. In this system, where the monolith column is incorporated into a column-switching valve, on-line digestion can take place at low pH (pH 2 or 4.5), which is a suitable mobile phase for direct MS interfacing.
Other polymeric monoliths were based upon the thermally initiated in situ copolymerization of methylstyrene and 1,2-bis(p-vinylphenyl)ethane in capillaries. By controlling the polymerization time, the porosity could be modulated and finetuned to match the application. Butyl-methacrylate, butyl-acrylate, and lauryl methacrylate monolithic columns could be synthesized, and depending upon the porogenic solvent mixture that was used, the pore size and column efficiency (in the CEC mode) could be controlled. Weak-anion-exchange polymeric monoliths were polymerized in situ in 4.6- and 1.0-mm i.d. columns. Other polyethylene glycol-dimethacrylate monoliths were further treated with polyethyleneimine and afterwards quaternized with methyl iodide to form an anion exchanger. Common anions could be separated quickly and efficiently. Other methacrylate monomers were prepared with surface grafting of presynthesized telomers with well defined lengths made from vinyl monomers with different functionality. It appears that there are many ways to prepared polymeric monoliths and, unlike silica monoliths, most of them are not protected as intellectual property.
In addition to silica and polymeric-based monoliths, other starting materials can be used to prepare monolith phases. At HPLC 2007, a report of zirconia-based monoliths prepared inside of capillaries both coated onto a silica monolith and as a pure monolith showed some advantages. For example, electroosmotic flow in CEC was quite different for zirconia, and the pH value of the background electrolyte in the pure zirconia phase could be adjusted higher than with a silica-based monolith. Because phosphates have a high affinity for zirconia, a hydrophobic phosphate derivative was grafted onto the surface of the monolith to form a reversed-phase functional group. Another group reported on the use of a carbon-based monolith that was resistant to swelling and hydrolysis and also possessed properties of conductivity that could be useful in electromodulated LC.
A most interesting application of monoliths was described in a poster presented by a group from Dublin City University in which photochromic ligands (spiropyran and its derivatives) were attached to silica- and polymeric monoliths. These photochromic dyes change their structure upon absorption of light at a certain wavelengths. In their common form, the spiropyran is colorless and uncharged. It can generate a highly colored merocyanine zwitterion in the presence of polar solvents, at elevated temperature and by exposure to UV radiation at a wavelength of approximately 370 nm. A visible green light (524 nm) switches the spiropyran back into the uncharged form. The zwitterion form can act as a metal chelating agent and also exhibit ion-exchange interactions. In a miniaturized chromatographic device, these authors were able to preconcentrate cations followed by instantaneous elution by irradiating the stationary phase with a green LED. The column can then be switched back into the zwitterion form by irradiating it with a UV LED (370 nm).
Monolith columns are starting to solve real-world applications problems: the analysis of tocopherols in vegetable oils by CEC using methacrylate ester-based monolith columns; lactoferrin in milk; various proteins (on polymeric monoliths); protein tryptic digests (mostly on silica monoliths); anabolic steroids in animal feed; illicit drugs on banknotes and in treated waters; nitroaromatic compounds; synthetic polymers; and various pharmaceuticals — to name a few.
Sub-2-μm columns/UHPLC: Under the technology category (Table I), the third most covered topic was the sub-2μm columns that were intertwined with papers on UHPLC. Other terms used in this area were fast LC, ultrafast LC, rapid-resolution LC, high-speed LC, ultra-speed LC, high-throughput LC, as well as a few others. No wonder people are confused with the nomenclature in this sub-2-μm world. In reality, the sub-2-μm particles are the normal progression of the decrease in packed column particle sizes that has occurred over the years from 10 μm in the 1970s to 5 μm in the 1980s and 1990s to the 3–3.5 μm particles in the 1990s and early 2000s. There is nothing magic about the use of these particles except that to take full advantage of them, especially when they are packed into very short (less than 30 mm) and small internal diameter (less than 2.0 mm) columns, one must pay more attention to such experimental parameters as extra column effects and gradient delay volumes.
At HPLC 2007, mean particles sizes of 1.5, 1.7, 1.8, 1.9, and 2.0 μm were discussed, many in oral and poster presentations, in tutorials and short courses and at vendor workshops and exhibition booths. So, participants had lots of exposure to this technology during the week.
Several practical and theoretical papers discussed the merits and disadvantages of narrow, wide and engineered particle size distributions with the sub-2-μm particles. At HPLC 2007, more work on this subject was reported from the laboratory of G. deSmet at the Free University of Brussels, Belgium (8), who studied the relationship between particle size distribution and the kinetic performance of packed columns and, basically, their latest study was in the agreement with the early work of Verzele and DeWaele (9). The method of measurement of particle size distribution is an important factor: number-size and weighted volume-distributions give different results. The number of fines in the distribution has a powerful effect on the column permeability because of the inversely proportional relationship between pressure and particle size (~1/dp2).
There were many applications examples shown where HPLC methods performed on older 3- and 5-μm columns were easily converted to the sub-2-μm columns with greatly reduced analysis times but with equivalent results. The high speeds shown with short sub-2-μm columns also can prove useful as the second column set for comprehensive- and 2-D LC. One of the more notable applications of sub-2-μm columns was the automated analysis of 21 protein hydrolysate amino acids using o-phthaldialdehyde (OPA) and 9-fluorenylmethyl chloroformate (FMOC) precolumn derivatization in just over 6 min. Other examples of very fast separations (2 min or less) were shown for vitamins, polynuclear aromatic hydrocarbons, endocannabinoids, metered dose inhalers, artemisinine and its derivatives, phenolics, pravastatin and related substances, oxytetracycline antibacterials, and anthocyanins to name a few.
Chiral separations: Chiral chromatography was another relatively hot area of activity. Although I did not tabulate the data here, after reversed-phase chromatography, chiral chromatography had the highest number of presentations. There were oral and poster sessions devoted to this mode of HPLC. Ever since the U.S. Food and Drug Administration declared that certain drugs with a chiral center should be enantiomerically pure, chiral separations have been receiving increased attention. In my recent survey (10), chiral chromatography has seen a substantial growth in the last decade.
Chiral compounds can be separated by HPLC, aqueous and nonaqueous CE and other electro-driven technologies, and SFC. SFC and simulated moving bed (SMB) chromatography have found a unique niche in the pharmaceutical industry in the preparation of pure enantiomers, especially on a large-scale basis.
In the past, chiral column selection has usually involved scouting multiple chiral stationary phases (CSPs) until one is found to resolve the sample. Although automated screen systems and method development software to screen combinations of mobile phases and these multiple CSPs are available to help out, it is still a tedious process. The chemists at Daicel (Tokyo, Japan) have strived to make the process simpler and have developed more "universal" chiral columns. They have improved their polysaccharide-based phases by immobilizing them on silica gel. The immobilized CSPs can now be used with a variety of solvents that limited their application in the past. Unusual mobile phases such as tetrahydrofuran, ethyl acetate, and chlorinated solvents can now be used for enhancing enantioselective recognition. With three columns in the Chiralpak series, the company claims that they can handle over 90% of the applications. Additional poster papers used this same approach to synthesize and resolve enantiomers by SFC and were compared with the Chiralpak columns.
In the past, many chiral columns could be used in the normal phase only. New types of chiral chemistries presented at HPLC 2007 were based upon phenylcarbamate derivatives of cellulose and amylase that could be used in normal phase, polar organic mobile phases, and reversed-phase eluents as well as SFC. In addition, silica monoliths have been functionalized by either coating or covalently immobilizing the chiral selector onto the surface.
Other chiral approaches reported at HPLC 2007 involved Pirkle phases, ligand exchange, various types of unmodified and modified cyclodextrins phases, immobilized quinine, macrocyclic glycopeptides phases, and alpha glycoprotein phases.
The measurement of chiral drugs in biological fluids usually involves an extraction followed by chiral separation or the coupling of a restricted access medium (RAM) column and a chiral column. A poster paper by G. Felix of the CNRS (Marseille, France) described a single column that could accomplish this separation. A combined RAM–chiral column was described in which the protein in the biological fluid (for example, plasma) was excluded by virtue of a very small pore on the silica packing, while the smaller chiral compounds could diffuse into the pore and interact with a CSP. After the protein was eluted, a step gradient was used to elute the chiral compounds.
Other stationary phases: Researchers now seem to be developing phases that are useful outside of the regular modes of reversed phase, ion exchange, size exclusion, and normal phase. Separation modes that were unknown (or unused) a few years ago are starting to appear in presentations — HILIC, mixed-mode separations, aqueous normal phase, ion exclusion, and separations based upon physical forces (not chemical) to name a few. I have selected a few examples of some of these newer modes to illustrate this phenomenon.
HILIC: Probably the biggest interest in these submodes has been in HILIC. HILIC is a separation technique for highly polar analytes that gets around some of the problems associated with reversed-phase chromatography such as low retention or phase collapse (dewetting). HILIC uses a polar stationary phase such as bare silica gel or polar-bonded phase (for example, diol) and requires a high percentage of a nonpolar mobile phase, similar to the requirements for normal-phase chromatography. However, unlike normal phase, which uses nonpolar solvents like hexane and methylene chloride and tries to exclude water from the mobile phase, HILIC requires some water in the mobile phase to maintain a stagnant enriched water layer on the packing surface into which analytes can partition selectively. In addition, water-miscible organic solvents are used. In HILIC, polar analytes are well retained and are eluted in order of increasing hydrophilicity.
David McCalley of the University of the West of England, Bristol, U.K. presented a lecture on the viability of HILIC for the analysis of basic pharmaceuticals. As much as 60–70% of all pharmaceuticals are basic, and HILIC is often appropriate. It can give better peak shapes and sometime convenient inversions of elution order relative to reversed-phase separations. In HILIC on bare silica, hydrophilic bases are often well retained; their retention and selectivity can be controlled by manipulation of the organic modifier concentration and the pH and ionic strength of the buffer. Acidic and neutral compounds are eluted earlier than the basic compounds. He showed that the loading capacity for these solutes could be an order of magnitude higher than in reversed-phase separations, if an appropriate buffer was chosen- a significant advantage.
He suggested conditions where the HILIC mechanism came into play:
- A polar stationary phase
- Mobile phase consists of greater than 2.5% water
- Mobile phase contains at least 75% acetonitrile
The suggested mechanism for the retention of basic compounds is complex on bare silica and could include retention in the water layer that is adsorbed on the silanols of the silica gel, adsorption on the water-deactivated silanols, ionic retention, and reversed-phase retention on siloxane groups. Since HILIC is performed in a highly organic mobile phase, the van Deemter curves are lower and flatter at high flow rates. Long columns of small particles (for example, superficially porous silica) can be used to generate very high efficiency. Some parameters to be aware of: the silica columns used in HILIC may not stand up at high (apparent) pH values, and the equilibration times when the solvent is changed may be longer than in reversed phase separations.
In an oral presentation later in the day, Carl Sanchez and colleagues of Phenomenex (Torrance, California) presented a systematic approach for method development in HILIC. In their study, they systematically investigated the effect of column chemistry, organic modifier and buffer pH on selectivity and retention for a model set of compounds. Three columns — one proprietary highly cross-linked diol column for HILIC, a zwitterionic column, and a bare silica gel column — were recommended since, in their experience, they have shown the largest differences in selectivity. The columns were then systematically screened by varying the organic modifier and buffer pH (pH range 3–6). The effect of ionic strength, alternative organic modifiers and gradient slope were investigated as well. They also looked at isocratic versus gradient methods and put forth a set of guidelines for optimal experimental parameters of retention (k), selectivity (α), resolution (Rs), and peak asymmetry (As) for MS methods. The effect of the organic solvent concentration was somewhat predictable but the other parameters had to be optimized separately. To achieve the optimum HILIC method, they recommended adjusting the experimental parameters following the order presented earlier. They, too, found that, after gradient elution, column equilibration time in HILIC is quite long.
A number of new HILIC phases were discussed at HPLC 2007, including a methacrylate-grafted silica particle, biporous adsorbents built from nanoslabs, various zwitterionic phases, triazol bonded phases, amide polar embedded phases, zirconia-coated silica, and PEG coated silica.
Mixed-mode phases: Mixed-mode stationary phases are not normally thought to be desirable in HPLC because mixed mechanisms can result, which are sometimes difficult to control. However, Michael Laemmerhofer of the University of Vienna, Austria used them to his advantage when developing a column for metabolite analysis. Because most metabolites are in a high polar urine matrix, it is difficult to extract polar metabolites of importance selectively. In addition, these matrix compounds cause ion suppression in MS detection. His solution was to develop a mixed-mode stationary phase (reversed phase and weak anion exchanger) that was bonded to silica. The stationary phase contained a long hydrocarbon chain with an embedded sulfur group plus a nitrogen-containing end group (two nitrogens are part of a 2.2.2-bicyclic octane, one is at the bridging position and one is on the connecting side chain in the 3-position). With this phase, he and his colleagues have been able to perform ion-exclusion, ion-exchange, reversed-phase, hydrophobic intereraction, and HILIC separations — a true multimodal column. In this study, the group studied ethanol consumption markers ethyl glucuronide, ethyl sulfate, and ethyl phosphate in urine samples. They performed validation studies of over 600 runs and found the column to be extremely stable. Another study reported in this paper was the human metabolism of the organophosphate pesticide chloroyrufis in cases of acute intoxication. HILIC seems to be a good approach for investigating polar metabolites in biofluids when using MS, which prefers to have a high organic mobile phase content.
Superficially porous particles: The superficially porous particles have been around for decades, having been the very first high-efficiency packing used in the late 1960s. In more recent years, the concept has been revisited several times with the nonporous silica (11) and Poroshell (12) columns, both useful for large biomolecules. In his oral presentation, David Bell of Sigma Aldrich Supelco (Bellefonte, Pennsylvania) discussed a similar product (Ascentis Express) based upon fused-core technology, in which the the solid core was only 1.7 μm in diameter; there was a porous layer of 0.5 μm around it, making the particle diameter 1.7 μm. As expected, the pressure versus flow rate curve was around halfway between that noted for a 1.7-μm particle and a 3.0-μm particle. A 100 mm × 4.6 mm column operating at a flow rate of 1.5 mL/min gave a pressure drop of 248 bar, well within the pressure capability of nearly all HPLC systems. Because the peaks are narrow, he recommended that users optimize the system volume for best performance. Columns of the superficially porous packings can be joined together for high plate counts. A 550 mm × 4.6 mm column operating at 7000 psi generated about 100,000 plates. A separation of deutrated benzene from benzene was shown to illustrate the column performance. A poster paper elaborated on the performance of this column
HPLC 2008 is Next
The next major symposium in this series, the 32nd International Symposium on High Performance Liquid Phase Separations and Related Techniques (HPLC 2008), moves back to the United States and will be held in Baltimore, Maryland, May 10-16, 2008. This will be the second time this series has been held in Baltimore, and cochairmen of this upcoming event will be Prof. Georges Guiochon of the University of Tennessee and Oak Ridge National Lab and Prof. Steve Jacobson of Indiana University.
Ronald E. Majors
"Column Watch" Editor Ronald E. Majors is business development manager, Consumables and Accessories Business Unit, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to "Sample Prep Perspectives,"LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgc-mag.com
References
(1) R.E. Majors LCGC 24(9), 970–985 (2006).
(2) R.E. Majors, LCGC 18(11), 1122–1134 (2000).
(3) R.E. Majors, LCGC 1910), 1034–1048 (2001).
(4) R.E. Majors, LCGC 20(9), 830–841 (2002).
(5) R.E. Majors, LCGC 21(9), 872–887 (2003).
(6) R.E. Majors, LCGC 22(9), 870–882 (2004).
(7) R.E. Majors, LCGC 23(9), 989–1005 (2005).
(8) J. Billen, D. Guillarme, S. Rudaz, J-L Veuthey, H. Ritchie, B. Grady and G. Desmet, J. Chromatogr. 1 1161 (1-2), 224–233 (2007).
(9) M. Verzele and C. DeWaele, J. Chromatogr. 260, 13–21 (1983).
(10)R.E. Majors, LCGC 25(6), 532–546 (2007).
(11) T.J. Barder, P.J. Wohlman, C. Thrall, and P.D. DuBois, LCGC 15(10), 918–926 (1997).
(12) J.J. Kirkland, F. A. Truszkowski, C.H. Dilks, and G.S. Engel, J.Chromatogr. A 890(1), 3–13 (2000).
(13) G. Guiochon and M. Martin, J. Chromatogr. A 1090, 16 (2005).
(14) R.E. Majors, LCGC 23(10), 1074–1084 (2005).
(15) N. Tanaka, K. Horie, K. Miyamoto, T. Hara, H. Kobayashi, H. Morisaka, D. Tokuda, K. Koduki, S. Makino, O. Nunez, C. Yang, T. Ikegami, Kyoto Institute of Technology, Kyoto, Japan, Paper L17.01, HPLC 2007, Ghent, Belgium.
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












