ChromSoc Activities: Medals
Special Issues
A recollection of The Chromatographic Society’s medals and medallists over the years.
Chris Bevan1 and W. John Lough2, 1Meetings Organizer, The Chromatographic Society, UK, 2University of Sunderland, UK.
A recollection of The Chromatographic Society’s medals and medallists over the years.
To the worldwide chromatographic fraternity at large, The Chromatographic Society’s Martin Gold Medal and the Silver Jubilee Medal are undoubtedly the most visible and recognized activities of the Society. In these days of awards proliferation, they are renowned as being amongst the highest distinctions awarded for excellence in the separation sciences.
Figure 1: Clockwise from top left: Roger Smith receives his Jubilee Medal from President Derek Stevenson in 1998; Jack Henion, seen here with medal presenter,

John Lough (right) at Edinburgh, is awarded the Martin Medal in 2003; a glittering array of medal winners at the Microcolumn meeting in March; and the Martin Medal.
Perhaps to some this may be because they appear to have been around since the Society was formed. However, while they do have a long heritage, the facts do not quite bear this out.
The Society was founded in 1956 but the Martin Medal was not awarded until 1978, to Ernst Bayer and C.E.H. (Ted) Knapman. The Jubilee Medal - as its name implies - followed on four years later just before the Society’s Silver Jubilee in 1982. The first Jubilee Medals were awarded to R. Tijssen, K. Grob Jr, and P.J. Simmonds. When first instituted the Jubilee Medal was originally intended to be in recognition of the outstanding achievements of young up-andâcoming chromatographers in the middle of their career. However, this has now been partially modified and the requirements for each award have been defined more clearly:
The Martin Gold Medal is awarded to scientists who have made outstanding contributions to the advancement of separation science.
The Jubilee Silver Medal is awarded to younger separation scientists who have made major use of separation science in their own field, or to scientists who have made contributions of merit to a particular area of separation science.
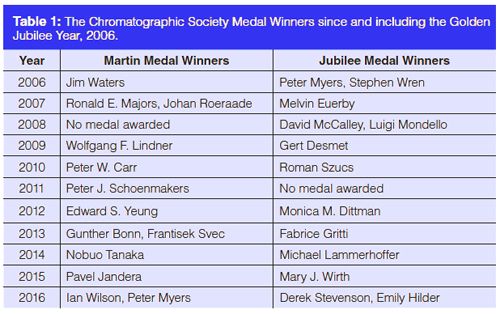
A complete list of medal winners may be viewed on the Society’s website (www.chromsoc.com) Winners from 2006 are listed in Table 1.
As we write, the process for selecting medal winners for 2017 is underway. The process has always been thorough but nowadays it has become a protracted in-depth affair involving the votes of the members and examination of publications and CVs. Only in this way can we ensure that the medals are awarded to those scientists and technologists considered to be of the very highest quality by Chromsoc’s committee and its members.
Chris Bevan is a former Group Leader of the physicochemical characterization team at GSK and former President of The Chromatographic Society. He is now retired and works as Chromsoc’s Events Coordinator.
John Lough is a Reader in Pharmaceutical Analysis in the Sunderland Pharmacy School at the University of Sunderland, in Sunderland, UK, and a member of the Executive Committee of The Chromatographic Society.

Evaluating the Accuracy of Mass Spectrometry Spectral Databases
May 12th 2025Mass spectrometry (MS) can be effective in identifying unknown compounds, though this can be complicated if spectra is outside of known databases. Researchers aimed to test MS databases using electron–ionization (EI)–MS.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)













