Chromatography for Bioanalytical Chemists
This article looks at current practices in bioanalytical chemistry by examining and critically assessing the various parameters that can be altered to achieve high-speed results with high resolution in LC–MS applications. The decision to opt for gradient or isocratic elution is also discussed.
Bioanalytical chemistry — the quantification of chemical entities in biological matrices — is a dynamic science that is constantly evolving. It is a key activity within medical and pharmaceutical research both for drug discovery and development. The costs of developing drugs has increased significantly and the drive towards higher productivity and shorter development times is crucial in all the areas of the drug discovery process. Bioanalytical chemistry is no exception — more must be done in less time.
Fortunately, we have the tools to meet these demands. The now mature marriage between liquid chromatography (LC) and mass spectrometry (MS) is probably the most significant step forward in the last 20 years. Other techniques are still successfully used in niche applications, yet LC–MS is now the main approach in bioanalytical chemistry. The pharmaceutical industry regularly uses this technique in early discovery work to screen substances for metabolic and absorption properties, for example.
LC–MS can now provide screening information that was previously very time-consuming to obtain using other bioanalytical techniques and has thus been the main focus for the progress of bioanalytical chemistry. The analytical methodology is by necessity more or less generic — sample preparation is often done by precipitation and chromatography by fast gradients.
Within drug development work bioanalytical chemistry is vital for studying, for example, the in-vivo pharmacokinetic properties. Extensive studies are done for each drug and bioanalytical chemistry is called upon to establish robust and sensitive methods according to GxP standards. Analytical methods are tailored for each compound. In practice this means selective sample preparation optimized for each drug and often chromatography by isocratic elution to enable short cycle time and high resolution.
So what does all this mean for the chromatographic aspect of LC–MS?
What Do We Want From Chromatography?
When the LC–MS euphoria started it seemed like chromatography could be minimized or even excluded. However, the bioanalytical community woke up with a slight hangover in the mid-90s. Poorly separated samples resulted in variability in analyte response. The phenomena were reported as "ion suppression"1,2 — a matrix-induced reduction in analyte response. Later it was observed that one might also get matrix-induced enhancement of the response. It was also noted that, for example, coeluting metabolites might be converted back into the main compound.3 Methods for multiple analytes also benefit from separation as simultaneous measurement of several analytes reduces signal-to-noise (S/N) ratios. Hence, the importance of chromatographic separation in LC–MS method has been emphasized recently.2,3
So, what do we want from chromatography, considering the detection technology available and the demands of increased productivity? The first thing to point out is the need for retention. (This might appear ridiculously obvious, however, in a frightening number of LC–MS methods the analytes are almost not retained!) The analyte(s) should elute far from the void volume to reduce the risk of ion suppression2 and to obtain resolution. Secondly, the higher column efficiency the better because there is less risk of ion suppression and less time needed for selectivity optimization. Thirdly, the cycle times should be short. Although MS needs chromatographic resolution, its high selectivity needs to be translated into short cycle times to achieve the ever-increasing demand for throughput.
The goal is thus (not surprisingly) to get sufficient retention and highest resolution power within a short time frame. How fast is fast enough? Impressive fast separations are often reported in the literature. Yet, when reducing cycle time one reaches a point where sample logistics, sample preparation, data evaluation and reporting become a bottleneck. Where is the point beyond which further reduction in separation time is meaningless? This breaking point is probably different for discovery and development work. The more elaborate sample preparation schemes and stringent result acceptances criteria applied in development work soon become a bottleneck when separation time is reduced. In the discovery arena this is less of an issue and short separation times are appreciated more.
There is no workflow analysis reported that could provide a suggestion of how short the cycle time needs to be to move the bottle neck from chromatography to other steps in the analytical work — it is also dependent on how efficient the various steps are implemented in a laboratory. A wild guess? One minute for discovery and two minutes for development work...
Reducing Chromatographic Run Times
Several approaches have commonly been used for fast chromatography during the last decade. Below are some common routes presented and critically reviewed.
Short columns: It is obvious that separation time is reduced with shorter columns. This is simple and easy to implement. The price one pays is reduced resolution because of reduced plate numbers. The mathematics is trivial: half the column length means half the run time and half the number of plates. However, reducing the column length while keeping everything else constant is the most expensive route in terms of efficiency loss.
Reduced retention: Another evident possibility of saving time is to reduce retention. This makes sense to some extent. However, there is no gain in going down to a capacity factor (k') less then three — the sacrifice in resolution and risk of ion suppression is then too large.
High flow-rates: Flow-rates have been dealt with in chromatographic textbooks for many decades and "standard" flow-rates have been established for common column diameters. These "standard" flow-rates correspond to (more or less) highest column efficiency. However, it is often advantageous to use higher than normal flow-rates to gain time.4–9 Doubling the flow-rate saves just as much time as using a column with half the length. Increasing flow-rate also means less reduction of plate number than reducing column length. Usually high flow-rate also translates into high backpressure. The latter will set the practical limit for high flow-rates.
Small particles: Columns containing very small particle size materials are fundamentally the optimal solution for chromatography with short separation times.6 It is very challenging to produce these columns, however, they are becoming more common on the marketplace. Currently there are columns with particle sizes of 2 μm being established on the market. This approach does, however, result in high pressures. Yet, acceptable column efficiencies can be achieved with very short columns. This strategy will be compared with longer columns of 5 μm particle size operating at high flow-rates later in this article.
It has been stated that smaller particle columns are more likely to be blocked,6 — a potential problem with relatively dirty samples of biological origin. There are still no data that support this concern. If you are concerned with column blocking you should probably stay with particles larger than 3 μm.
High temperatures: Temperature is an important chromatographic parameter. Yet, it has not, until recently, been given much attention. With separation times in mind there is a lot to gain by increasing temperature.7,10–12 The viscosity is reduced which gives lower backpressure and higher efficiencies.13–16 The latter is especially noticeable at higher flow-rates. A higher temperature allows faster chromatography with increased reduced plate numbers and reduced backpressure. Limitations stem from column and analyte stability.12,15–18 Silica-based columns are generally not stable at temperatures above 80 °C. Analyte stability is probably less of a problem as the separation times are short.18 Higher temperatures (> 50 °C) also require mobile phases to be preheated immediately before the column.10,12,19–21 The design of mobile phase preheating devices is crucial to prevent band broadening because of temperature gradients or dead volumes.20 When going up to higher temperatures one will also notice that retention is reduced. For reversed-phase separation this translates to eluents with lower concentration of organic solvents (which may reduce MS ionization efficiency).
With all these benefits and limitations what should the practitioner settle for? Let us start by acknowledging that controlled temperature improves chromatographic reproducibility — adding a column thermostat to your LC–MS system is good practice. The cautious analyst would then probably arrive at 40–50 °C where column and analyte stability is not much of an issue and preheating of mobile phases is not a must, making the hardware requirements less challenging. The more adventurous would go down the 60–80 °C road. Once again, work practices need to be assessed to find the mode that is the best fit for the operation at hand.
Monolithic columns: The introduction of totally porous silica columns (marked as monolithic columns) has gained considerable attention recently.22–29 The benefit is clear, high flow-rates can be applied with very limited loss of efficiency and increase in backpressure. This is indeed a promising technique. Still, narrow bore columns that would allow these materials to be fully used in a practical format seem difficult to manufacture.29 In addition, it seems that the materials currently available have more problems with tailing chromatographic peaks than conventional columns.22,25,29 Totally porous columns are an interesting step forward that hopefully will mature to become a true and practical improvement for fast separation.
Ultra-high pressure: Theoretically pressure sets the limit for speed and resolution.30 Just imagine that we could run a 2 × 500 mm column with 1 μm particles at a flow-rate of 1 mL/min. Pressure sets limits to the column lengths, flow-rates and particle sizes that we may use. Going to higher pressures (by any of these routes) is a matter of convenience and capability. In other words, which pressure one feels comfortable to work with from a practical point of view and the pressure limit of the pumping system. Pressure has always been the ultimate limit for chromatography. This has perhaps not been realized by all chromatographers but as the commercial pumping systems that can deliver very high pressures are being introduced,31 vendors are emphasizing this fundamental (which is probably more convincing than the chromatographic text books have ever done). A spin-off from this insight is that the organic solvent used for reversed-phase mobile phase should have low viscosity — acetonitrile is the preferred solvent.
Agreeing on a manageable upper pressure limit for everyday use is a decision each laboratory needs to make based on its experience and equipment. This will be an essential ground for deciding on column format.
Experimental
Chromatography was done using an Agilent 1100 system with a high-pressure mixing pump, well-plate autosampler with mobile phase preheater. When column efficiency was studied a diode array (DA) detector with 1.7 μL flowcell was used. The comparison of sensitivity was done with a 1100MSD with an electron spray ionization (ESI) and atmospheric pressure chemical ionization (APCI) interface.
Plate number was calculated for different column lengths, flow-rates and column temperatures from plate heights that was determined for flow-rates between 0.2–2.5 mL/min for 50 × 2.1 mm Hypersil C18 Gold columns packed with 1.9, 3 or 5 μm. Plate heights were calculated as N/L, where N is the plate number and L the column length. The determinations were made using phenytoin as model substances. The model systems were eluted with mixtures of acetonitile and buffer (5 mM formic acid and 10 mM ammonium formate) giving a capacity factor between 4 and 6. The found plate heights was fitted to the Knox function (H = Au0.33 + B/u + Cu, where u is linear flow-rate) using reduced parameters to allow plate heights to be estimated for all flow-rates.
The mass spectrometric sensitivity study was done by monitoring the M+ H+ ions of carbamazepine.
Evaluating The Options
There are an endless number of studies of the effects of particle sizes, column diameters, column lengths, flow-rates, column temperatures, pressure — and the myriad combinations. Analytical scientists and vendors are heading in many different directions. The working chromatographer needs to find the right compromise using this flood of information. The column catalogue is on your desk and you need to decide on the column format.
How then does the chromatographer on the street (sorry, in the lab) get the "best bang for his buck"? In my view the "most bang for the buck" translates in chromatographic terms to the highest plate number with the highest tolerable backpressure and acceptable cycle time. So, with a target cycle time in mind your "bucks" are psi/bar and the "bang" is plate number. This evaluation is done with the mind of someone who needs a certain cycle time and wants to achieve highest efficiency with the pressure that he or she can tolerate — and other practical constraints of personal flavour (practical column temperature and minimal particle size).
The Right Retention
What then is "sufficient retention"? It is not possible to define strict rules. In the author's opinion the retention volume should be more than three times the void volume. In this work a capacity factor (k') of four has been chosen as the target.
The capacity factor is by many LC users vaguely perceived as a more generic expression of retention ("a bit more scientific then retention time but almost the same thing"). In this context it is important to realize that for a given capacity factor one may actually obtain whatever retention time one wants by varying column length and flow-rate. A short retention time does not necessarily imply a low capacity factor.
Objective
In this article the first scenario considered is the quantification of one compound, or a small number of closely eluted compounds, by isocratic elution. Gradient elution will be discussed later.
The starting point is to estimate an acceptable retention time and capacity factor. In this study this is assumed to be two min and a capacity factor of four. These two requirements result in only one demand on the column dimensions and flow-rates — the column dead time (t0) should be 2.0/(4 + 1) = 0.4 min. (One may find somewhat different retention and capacity factor being appropriate for a particular objective. The investigation presented here should, however, still be useful.)
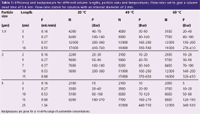
Table 1: Efficiency and backpressure for different column lengths, particle sizes and temperatures. Flow-rates set to give a column dead time of 0.4 min. Flow-rates stated for columns with an internal diameter of 2 mm.
The objective of this study is to compare different combinations of column lengths, temperatures, flow-rates and particle sizes that all have a t0 of 0.4 min. The simple question is "Given that I can live with a pressure of X bar — how do I get the highest plate numbers?"
Results
The results of the study are summarized in Table 1. The flow-rates are given for columns with an internal diameter (i.d.) of 2 mm (this needs to be rescaled if other dimensions are used) and have been adjusted for each column length to have a column dead time (t0) of 0.4 min. Some key observations are given below.
Fast separations and sufficient retention can be achieved with long columns: If you ever thought that you require short columns for fast separation — maybe you should think about high flow-rates instead.
Higher temperatures are effective: Backpressure is reduced and plate numbers increase. The latter means that longer columns and higher flow-rates can be used — giving even higher efficiencies.
The efficiency gained by smaller particle sizes is partly offset by the shorter column length: The difference in resolution between columns with different particle sizes is significant but limited when comparing set-ups giving the same backpressure (i.e., different column lengths). This is mainly because longer column and higher flow-rates may not be used with smaller particles on account of the higher backpressures.
Theory into Practice
This example demonstrates the benefit of high temperatures and high flow-rates for fast separations. So how does this work in practice?
Higher temperatures are mainly a matter of finding a column oven that fits the instrumentation without adding more dead volume. For temperatures above 40 °C a mobile phase preheater also needs to be added.
High flow-rates raise issues regarding instrument backpressure (caused by capillaries, connections, detectors etc.) and detector compatibility. The simplest solution is to use columns with an i.d. of 1–2 mm. The high linear flow-rates (cm/min) then translate to moderate volumetric flow-rates (mL/min). One might perceive a problem with extra band broadening because of dead volumes in capillaries etc. with, for example, a 1 mm column. However, it is only necessary to pay attention to dead volumes when peak volumes (peak width in time multiplied by volumetric flow-rates) are small. Considering the relatively high flow-rates suggested here dead volumes would thus be less of a problem.
To minimize system backpressure it is also useful to select capillaries with appropriate internal diameters. Detector compatibility is more dependent on instrument. With MS detection the flow-rates tolerated depend on the type of interface. Early electrospray ionization (ESI) interfaces could not tolerate higher flow-rates. You were then forced to split the flow from the column. More modern interfaces can, however, tolerate high flow-rates (> 0.5 mL/min). APCI interfaces have always worked well at higher flow-rates. On the contrary APCI performance at low flow-rate may be poor. APCI also behaves as a mass sensitive detector and a larger column accepting larger injection volumes is preferred.
When possible it is convenient to standardize on a chromatographic set-up with a flow-rate that is compatible with both electrospray and APCI to allow fast changeover and method development.
How About Sensitivity?
The limit of quantification is in many instances crucial for the analysis. It has been claimed that higher flow-rates will lead to lower sensitivity.9 This is true if everything is held constant as the flow-rate is increased. However, to make a fair comparison of the S/N ratios for various column ratios, lengths, flow-rates etc., several factors need to be taken into account. The tolerable injection volume depends on the peak width in volume, which is different for different column lengths and flow-rates. The data collection rate needs to be faster with narrower peak in time — and the baseline noise increases with the collection rate. Finally, some detectors, such as electrospray MS (ESMS), are close to concentration dependent whereas, for example, APCI is mass flow dependent.

Table 2: Signalâtoânoise (S/N) ratio for different chromatographic setâups. Signal and S/N ratio set to unity for 4 x 30 mm column.
A theoretical and real case comparison using carbamazepine between two relevant set-ups with very different linear flow-rates for isocratic elution is shown in Table 2. The volumetric flow-rates are set to achieve similar retention times with the different column dimensions. The comparison is built on an injection volume that is proportional to peak width in volume and a data collection rate that gives a fixed number of data points for a chromatographic peak. For the theoretical comparison it assumed that the noise is proportional to the square root of the sampling rate. This example indicates that chromatography with high linear flows should not significantly reduce S/N ratios, even if narrower columns need to be used to arrive at practical flow-rates.
Gradient Elution
So far this evaluation has been referring to isocratic elution. This mode has several advantages when single or closely related analytes are to be determined in a development arena. With isocratic elution a separation is simple to reproduce on systems with different pump and autosampler configuration (i.e. different delay volumes) and no time is needed for reequilibration. It is also often easier to separate closely related analytes by isocratic elution.
Gradients are still the obvious choice in a discovery environment where the chromatographic condition set-up should work for a large number of different applications. It is also beneficial to use a gradient when several analytes with larger differences in retention need to be determined in one chromatographic run. To allow gradient elution and reequilibration with the same cycle time as isocratic elution you need column dimensions and flow-rates that give a column dead time about 4–6 times less than for isocratic elution. If column dead time is set at 0.08 min we arrive at the efficiencies and backpressures shown in Table 3. The main difference is that the benefit of going down in particle size increases compared with the previous isocratic scenario.
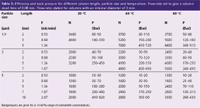
Table 3: Efficiency and back pressure for different column length, particle size and temperature. Flow-rate set to give a column dead time of 0.08 min. Flow-rates stated for columns with an internal diameter of 2 mm.
With gradients it is also necessary to select instrumentation with minimal delay volume (the volume between the point where the solvents meet and the column head) as this can significantly contribute to the cycle time. Furthermore, the chromatographic peaks will be very narrow in time, calling for very high data sampling rates that may lead to poor S/N rates. In essence, short cycle times are much more challenging for the instrumentation when gradients are used. When short cycle times and/or robustness are vital gradients should only be a for-cause mode — isocratic separations are easier to reproduce and less challenging for instrumentation.
Chromatographic Self-Assessment
To select the correct chromatographic format suitable for bioanalytical analysis you need to ask the following questions.
- Do you need gradient elution?
Gradient will be more demanding in many ways — do you need it?
- What's the maximum pressure you feel comfortable with?
Do you like to grab any polyetheretherketone (PEEK) fitting and tubing in the lab — well then you should probably not work at pressures higher then 200 bar. Are you happy to work with steel tubings and well-matched fittings — you are then probably able to go up to 400 bar. If you are willing to invest in specialized ultra high pressure pumping systems you can get closer to 600 bar.
- What column dead time suits you?
When you aim for isocratic elution and cycle times of 2–3 min a dead time of 0.4–0.6 min is a good choice. With gradient and similar cycle times you need dead times around 0.1 min. When you are willing to increase the cycle time you can increase the dead time accordingly.
- How do you feel about column thermostating?
Column thermostating makes sense assuming one can maintain low extra dead volumes. With any kind of column an oven temperature of 40 °C is easy to implement. Higher temperatures will need a well-designed mobile phase heater installed immediately before the column.
- Are you concerned about the blocking of small particle columns?
Considering the lack of published data there are hopefully some experiences in your laboratory that can tell you if small particles are troublesome.
- Which volumetric flow-rates to you like to work with?
Larger flow-rates put less demands on hardware and tolerate larger extra dead volumes because of capillaries and fittings. However, if you want to avoid splitting the flow into the MS you may need to work with lower flow-rates for some MS interfaces. In the results presented here flow-rates have been given for columns with an internal diameter of 2 mm. When you want to work at other flow-rates — select another column dimension and rescale the flow-rate accordingly.
Practical options
When you have done the self assessment above you can hopefully use the data given to arrive at a column and conditions suiting you. Where you end up will probably depend on what is expected by your laboratory and actual work practice. In a laboratory where dedicated methods should work day-and-night on every instrument (possibly even transferred to other laboratories) and in the hands of every analyst it makes sense to try to aim for isocratic elution. A conservative approach would be 2 × 100 mm columns with 5 μm particles using a flow-rate of 0.6 mL/min and a column temperature of 40 °C. The more adventurous user might use the same column dimensions but with 3 μm particles and run it at 60 °C. Considering the needs the gain of going to smaller particles and/or very high pressure and/or higher temperatures is, however, maybe not worth its price in terms of reduced robustness.
In instances where gradient elution is a must, cycle time has to be very short, high efficiency is crucial and methods are not transferred, it could make more sense to push the limits. What's the right compromise between robustness and performance? At one end you'll find 2 × 50 mm columns with 5 μm run at 40 °C, 1.3 mL/min and approximately 100 bar. At the other end the same flow-rate and column dimensions but packed with 2 μm particles and run at 60 °C giving a backpressure of 300–500 bar. The difference? Plate numbers of 2000 or 8400.
Conclusion
It is sometimes believed that chromatographic resolution can and needs to be sacrificed to achieve short separation times in the era of mass spectrometric detection. Hopefully, this article has shown that high chromatographic resolution (a product of retention and efficiency) can be obtained even with short cycle times.
The data presented here should allow you to select the optimal combination of column dimensions, particle size, flow-rates and temperatures — based on the work practice and requirements of your laboratory.
Before summarizing this work a word of concern should be expressed. Chromatographic expertise seems to be diminishing among bioanalytical workers. Fundamentals such as capacity factors, efficiency and resolution are getting less well understood. Good habits regarding capillaries, fittings, sample solvent, mobile phase selection and preparation are also often forgotten. The situation is often very obvious — a lot of the separation efficiencies strived for are lost, for example, because of inappropriate sample solvents or extra column volumes found in spaghetti-style chromatographic set-ups commonly seen in LC–MS laboratories. State of the art instrumentations and columns is not enough — you still need to understand chromatography.
Acknowledgements
The creative discussions with Martin Ahnoff regarding practical and theoretical aspects of chromatography have been a valuable inspiration for this work. The sponsorship provided by Helene Stenhoff is most appreciated. Both Martin Ahnoff and Helene Stenhoff work at AstraZeneca R&D, Mölndal, Sweden.
Niklas Magnell is an associate principal scientist at development bioanalytical chemistry at AstraZeneca R&D Mölndal. He obtained his PhD at Uppsala University in 1992 and his post doctoral at Roche, Switzerland between 1993 and 1994. He worked as an analytical scientist at Pharmacia, Sweden from 1994–1996 and then continued as an application specialist at Agilent Technologies from 1996 until 2001. His main research interest is bioanalytical method development strategies.
References
1. R. Bonfiglio et al., Rapid Commun. Mass. Spec., 13, 1175 (1999).
2. B.K. Matuszewski et al., Anal. Chem. , 70, 882 (1998).
3. M. Jemal and Y-Q. Xia, Rapid Commun. Mass. Spec. , 13, 97 (1999).
4. I.M. Mutton, Chromatographia, 47(5/6), 291 (1998).
5. M.J. Berna et al., Anal. Chem. Acta, 509, 1 (2004).
6. J.J. Kirkland, J. Chromatogr. Sci., 38, 535 (2000).
7. X. Yang et al., J. Chromatogr. A, 1079, 213 (2005).
8. Y.-F. Cheng et al., Rapid Commun. Mass. Spec., 15, 141 (2001).
9. A.T. Murphy et al., Rapid Commun. Mass. Spec., 16, 537 (2002).
10. T. Welsch et al., J. Chromatogr. A, 728, 299 (1996).
11. D.V. McCalley, J. Chromatogr. A, 902, 311 (2000).
12. D. Guillarme et al., J. Chromatogr. A, 1052, 39 (2004).
13. G. Sheng et al., J. Micro. Sep., 9, 63 (1997).
14. P. Molander et al., J. Chromatogr. A, 847, 59 (1999).
15. T. Greibrokk and T. Andersen, J. Chromatogr. A, 1000, 743 (2003).
16. B. Yan et al., Anal. Chem., 72, 1253 (2000).
17. S.J. Marin et al., J. Chromatogr. A, 1030, 255 (2004).
18. J.D. Thompson and P.W. Carr, Anal. Cheml., 74, 1017 (2002).
19. G.P. Rozing and H. Goetz, J. Chromatogr., 479, 3, (1989).
20. R.G. Wolcott et al., J. Chromatogr. A, 869, 211 (2000).
21. J.D. Thompson et al., Anal. Chem., 73, 3340 (2001).
22. N. Wu et al., Anal. Chim. Acta, 523, 149 (2004).
23. K. Mistry and N. Grinberg, J. Liq. Chromatogr. & Rel. Techn., 28, 1055 (2005).
24. B. Bidlingmeyer, J. Chromatogr. A, 832, 11 (1999).
25. D.V. McCalley, J. Chromatogr. A, 965, 51 (2002).
26. J.-T. Wu et al., Rapid Commun. Mass. Spec., 15 (2001).
27. N. Tanaka, Anal. Chem., 1 August, 420A (2001).
28. H. Zeng et al., J. Chromatogr. B, 788, 331 (2003).
29. N.C. van de Merbel and H. Poelman, J. Pharm, Biomed. Anal., 33, 495 (2003).
30. F.D. Antia and C. Horvath, J. Chromatogr., 435, 1 (1988).
31. M.I. Churchwell, J. Chromatogr. B, 825, 134 (2005).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











