Choosing the Right HPLC Stationary Phase
A guide to selecting the correct HPLC stationary phase.
An excerpt from LCGC's e-learning tutorial on HPLC stationary phases at CHROMacademy.com
There is a bewildering array of stationary-phase choices available for reversed-phase high performance liquid chromatography (HPLC), and even within each phase designation (such as "C18") the selectivity of each phase can vary widely.
Let's be honest, a lot of our method development is carried out using trial and error, typically based on phases that have worked for us in the past or are the "new best thing" from our favourite manufacturer. Even those of us with advanced "screening" platforms containing arrays of carefully considered orthogonal chemistries and computer-optimized eluent design systems, sometimes have to resort to "chromatographer's instinct".
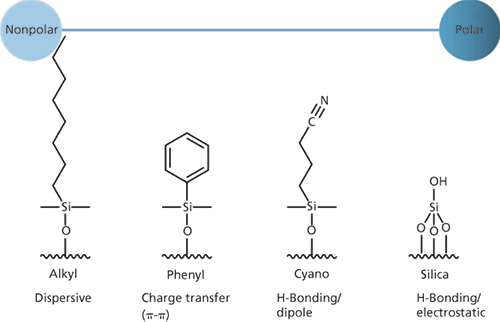
Figure 1: Retention mechanisms of some common reversed-phase ligands and residual silica surface species.
Retention in reversed-phase HPLC is typically based on an equilibrium between the analyte, the mobile phase, and the bonded stationary phase (C18, for example), and the nature and accessibility of the silica surface onto which the ligand is bonded. The chemistry of the bonded phase, the nature of the silica surface treatment, and the surface accessibility all need to be considered and classified to properly understand the retention mechanisms that are influencing a separation, which will then influence the initial column choice or method optimization.
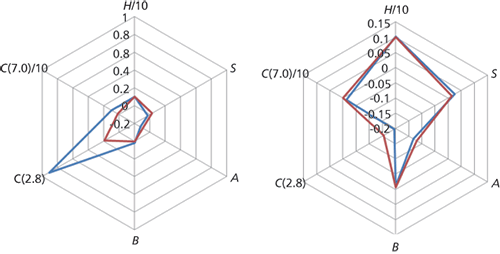
Figure 2: Radar plots of similar (right) and orthogonal (left) stationary phases based on data from the PQRI database, which in turn is based on the hydrophobic subtraction model.
Dispersive interactions are predominant in most reversed-phase separations, especially those using unmodified alkyl ligands (C18, C8, C4), and retention will be proportional to the hydrophobicity of the analyte. Charge transfer (or π–π) interactions are in play when aromatic or unsaturated phases or analytes are analyzed. Dipole-hydrogen bonding interactions are important for the retention of polar compounds, and stationary phases such as "cyano" enhance this type of retention. Electrostatic interactions occur between ionized sites on the analyte molecule and the silica surface, and in most cases are caused by ionized residual silanol groups.
Many column-classification systems exist, based on the use of various chemical test probes that are known to describe unique characteristics of a stationary phase, and one very useful example is the Product Quality Research Institute (PQRI) database hosted within the USP website (http://www.usp.org/app/USPNF/columnsDB.html). This database uses the hydrophobic subtraction model of retention (1,2) to describe phases based on hydrophobicity (H), the ability to distinguish between analytes of similar hydrophobicity but different shape or hydrodynamic volume, shape selectivity (S), hydrogen bonding (the ability to act as a Lewis acid [A] or a Lewis base [B]), and electrostatic interaction (C) at pH 7.0 (total silanol activity) and pH 2.8 (acidic silanol activity likely to cause tailing with polar or ionizable analytes). More unique or orthogonal phases tend to have larger values for S, B, and C (7.0). These large databases are useful to compare phase characteristics and "radar plots" are a very useful way to do this.
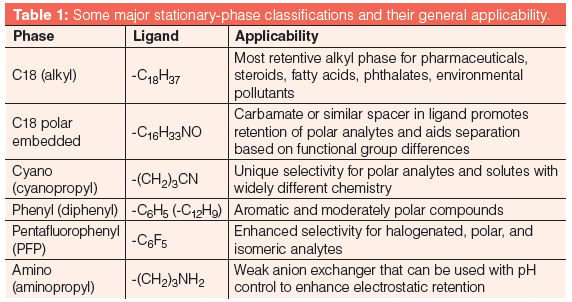
Table 1 shows a summary of the major classifications of phases currently available and their applicability.
References
(1) L.R. Snyder, J.W. Dolan, and P.W. Carr, J. Chromatogr. A 1060, 77–116 (2004).
(2) L.R. Snyder, J.W. Dolan, and P.W. Carr, Anal. Chem. 79, 3255–3261 (2007).

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








