Bioanalytical Analysis of Small-Molecule Drugs and Metabolites in Physiological Samples by LC–MS, Part 2: Sample Preparation
This article is the second of a series of four white papers on the bioanalysis of small-molecule drugs and metabolites by liquid chromatography–mass spectrometry (LC–MS). Part 1 was an overview of the fundamentals and regulations. This installment, part 2, is on sample preparation. Part 3 will cover method development, optimization, and best practices, and part 4 discusses method validation for regulated bioanalysis. This section provides an overview of sample preparation fundamentals, best practices, and modern trends. A sample preparation method development case study illustrates these practices and concepts.
Many factors need to be considered when developing sample preparation methods for bioanalysis. For a more in-depth discussion on sample preparation techniques, the reader is referred to textbooks on general applications in chromatography (1), pharmaceutical assays (2,3), and bioanalysis (4). Modern trends and practical aspects in sample preparation can also be found in numerous articles on “Sample Prep Perspectives” in the LCGC North America column by Douglas Raynie (5). Sample matrix, compounds, chemical properties, instrumentation, and desired limits of quantitation (LOQs) all affect method development decisions for liquid chromatography–mass spectrometry (LC–MS) analysis of complex physiological samples. Multiple options exist for sample cleanup, from more simple methods like filtration, pass-through solid-phase extraction (SPE), and liquid-liquid or supported liquid extraction (LLE and SLE), to more complex methods like hydrophobic interaction and mixed-mode ion-exchange SPE with bonded silica or polymeric sorbents. This paper aims to review emerging trends in sample preparation of biological samples such as blood, urine, and tissues, and sample preparation options based on their characteristics. Chemical properties such as the octanol–water partition coefficient (log P) and the acid dissociation constant (pKa) that affect sample preparation and method development are discussed. Automation strategies are summarized. A case study on sample preparation method development and experimental design for a drug in plasma is presented.
Method Development Considerations
First, the desired compounds, their required LOQs, and the instrumentation used for analysis should be determined. Will the method be a sensitive, quantitative method or a qualitative screen? Are there any relevant metabolites that need to be included? How much of the specimen is available? What is the stability of the analytes in and out of the matrix?
Knowing the properties of the biological matrix is important to determine the type of sample preparation required. For example, urine, blood, or tissues all have components that can cause ion suppression or enhancement, and can foul the LC column and LC–MS instrumentation (4).
Matrices
Whole blood is a commonly analyzed matrix. It is about 80% water. The remaining 20% is primarily red blood cells (43%), plasma (50%), proteins, fats, and sugars. Plasma is mainly composed of proteins like albumin, lipids, and globulins. Lipids and phospholipids in plasma can cause ion suppression and build-up on LC columns, resulting in clogging and reduced column lifetimes. Analytes can be bound to red blood cells or plasma proteins. Many analytes are bound to red blood cells that must be lysed during sample preparation for accurate quantitation. Water, acidic or basic buffers (ammonium chloride, trichloroacetic acid, zinc sulfate, perchloric acid), freezing, or centrifuging can lyse red blood cells. Acidic buffers or organic solvents (trichloroacetic acid, phosphoric acid, formic acid, zinc sulfate, methanol, ethanol, isopropanol) can disrupt plasma proteins (4,6).
Urine, another commonly analyzed matrix, is about 95% water. The main interferences that should be removed from urine during extraction are salts, urea, pigments, and creatinine. Many drugs and metabolites are enzymatically glucuronidated to aid in excretion from the body. These glucuronides can be measured directly, but they usually do not ionize well, and are more polar than their non-conjugated “free” drug or metabolite counterparts. Hydrolyzing conjugated metabolites to the “free” drug or metabolite increases sensitivity and simplifies method development. Hydrolysis can be done with acid or base, but these are not very selective, and usually require a pH adjustment prior to extraction. Enzyme hydrolysis with a natural or recombinant beta-glucuronidase enzyme is more selective. However, using an enzyme introduces proteins to your urine sample that should be removed before analysis (7).
Tissues require extensive sample clean-up. Commonly analyzed tissues include liver, brain, heart, and umbilical cord. Tissues contain blood vessels, arteries, veins, muscles, and other solid components. Tissues cannot be analyzed directly. They need to be homogenized and the analytes of interest extracted followed by sample preparation to remove inferences. Homogenization can be done with a blender, bead mill, tissue grinder, or bead ruptor. Samples can be homogenized in the presence of aqueous or organic solvent until they are liquified. The homogenate is usually centrifuged, and the supernatant is extracted for additional clean-up prior to LC–MS analysis (8).
Analyte Properties
Knowing the physicochemical properties of the target analytes can help determine the sample preparation procedure. Information on the structure, functional groups, polarity, pKa, log P, and stability is beneficial for selecting the appropriate approach and extraction parameters.
The log P indicates an analyte’s hydrophobicity. Analytes with a negative log P are more soluble in an aqueous solvent or more hydrophilic. Conversely, analytes with a positive log P are more soluble in an organic solvent or more hydrophobic. This is an important consideration when developing LLE, SLE, or hydrophobic interaction SPE methods. The distribution coefficient, log D, provides information on the solubility and hydrophobicity of ionized compounds (9).
Knowing the functional groups of the molecule can help determine whether it is an acid or a base. For instance, acidic drugs might have a sulfonic acid, carboxylic acid, or aromatic sulfonamide as part of their structures. Similarly, basic drugs might have phenols, imides, aliphatic amines, aromatic amines, or heterocyclic amines as part of their structures. Amphoteric compounds contain both acidic and basic functional groups, and have both a positive and a negative charge under certain pH conditions. Knowing a compound’s functional groups can aid in choosing possible sample preparation options (4).
The negative log of the acid dissociation constant, or pKa, is the pH where a functional group of an analyte is 50% ionized and 50% neutral (non-ionized). This value is particularly important when developing sample preparation methods as the pH of the sample should not be near the analyte’s pKa, because the compound would be in two different ionization states. An analyte is usually fully charged or fully neutral two units away from its pKa. This phenomenon is defined by the Henderson-Hasselback equation (2). For example, for a weak base with a pKa of 8, the analyte would be 100% positively charged two units below the pKa (pH 6 or lower) and 100% neutral two units above the pKa (pH 10 or higher). Similarly, for a weak acid with a pKa of 6, the analyte would be fully ionized and negatively charged two units above the pKa (pH 8 or higher) and neutral two units below the pKa (pH 4 or lower). For LLE, SLE, and hydrophobic interaction SPE, compounds should be neutral. For mixed-mode ion-exchange SPE, compounds should be charged (10).
Many resources are available to research compound properties. Chemicalize (11) is a website that provides compound information like log P, pKa, log D, chemical structure, solubility, and other properties. It does require a paid subscription. The Human Metabolome Database (12) is a free service with information for many drugs and metabolites. Pubmed and Google Scholar are resources for peer-reviewed publications. Multiple reference books can also be used to research compound properties, for example, Disposition of Toxic Drugs and Chemicals in Man (13).
Sample Preparation Options
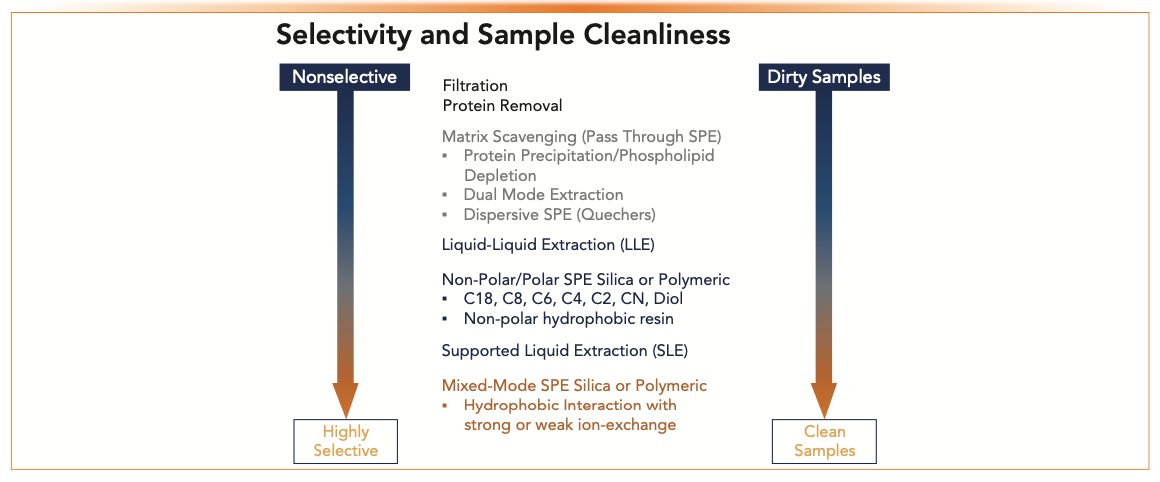
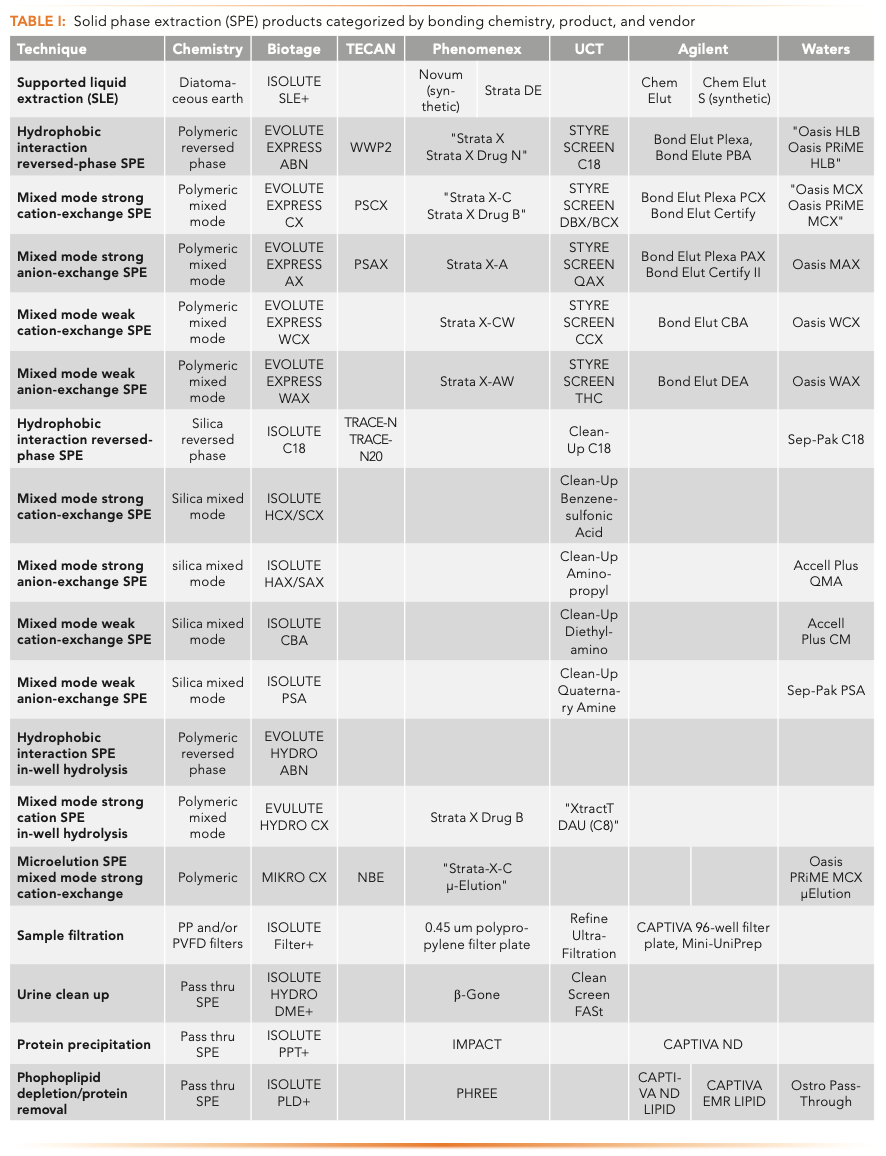
Figure 1 shows sample preparation techniques organized by increasing selectivity and increasing sample cleanliness. Sample preparation options can be selected based on assay requirements and analyte properties. Table I summarizes SPE product offerings from several manufacturers categorized by sample preparation options and sorbent chemistries. This is not meant to be an all-inclusive list, merely a sampling of what is available. The reader is encouraged to visit the company’s website for updated and detailed information. An overview of sample preparation options is described briefly below.
FIGURE 1: Selectivity vs. sample cleanliness using different sample preparation techniques.
Dilute and shoot methods, simply diluting the sample and centrifuging prior to analysis, yield the dirtiest samples. There is no clean-up involved, and all matrix components are still present in the sample, which can cause ion suppression or enhancement. Particulates can clog the LC column and contaminate the MS source.
Filtration of dilute and shoot samples can remove particulates from the sample. Samples can be passed through a syringe or vial filter or a filter plate for particulate removal prior to LC–MS analysis. These are usually 0.2 μm filters made of polytetrafluoroethylene (PTFE) or polyvinylidene fluoride (PVDF). An inline filter is usually placed pre-column, and can capture any particulates that would otherwise build up in the LC system. These filters should be changed frequently, as retained particulates accumulate and can leach out due to increased backpressure. LC guard columns can be used before the analytical column to retain components and increase LC column lifetime.
Protein removal is used for blood or plasma samples. This can be done manually or in a 96-well plate or cartridge. A crash solvent (such as acetonitrile or methanol) is added to the sample for a manual crash. Samples are mixed and centrifuged, and the supernatant is collected. A protein removal plate or cartridge removes proteins and filters the sample. The crash solvent is usually added first, then the sample is added, mixed, and vacuum or positive pressure is applied to collect the crashed sample. The precipitated proteins are retained while the rest of the sample flows through. Protein removal, using manual, plate, or cartridge protocols, only removes proteins, which are not the only endogenous component that cause matrix effects or interferences when performing LC–MS/MS quantitation (4,14).
Phospholipid depletion plates or cartridges are a simple pass-through SPE for plasma samples. This technique removes both proteins and phospholipids using a protocol similar to protein removal (add crash solvent, add sample, mix, and pass through sorbent). Precipitated proteins are retained by a filter, and phospholipids are retained by scavenger chemistry.
Dual mode extraction is a pass-through SPE technique that uses a hygroscopic salt layer and a scavenger chemistry to retain salts, urea, creatinine, and pigments from urine samples. Residual hydrolysis enzyme is also retained.
QuEChERS, a dispersive SPE, stands for “Quick, Easy, Cheap, Effective, Rugged, and Safe.” The extraction is done in a conical tube. Sample and acetonitrile are added to the tube. The tubes are then shaken, buffering salts are added, and the tube is centrifuged. The supernatant is transferred to a separate tube for evaporation, reconstitution, and analysis. This technique is popular for food and environmental analysis, but is sparingly used with biological samples. It is a laborious, manual process that cannot be easily automated (15).
LLE involves adding a water-immiscible organic solvent to an aqueous sample. The two phases are mixed and separated, usually by centrifugation. Analytes of interest partition into the organic phase, while most matrix components remain in the aqueous phase. Analytes should be hydrophobic, with a log P >1, and neutral for optimal results. This is a selective, cost-effective technique that yields clean extracts. However, emulsions may be present that make separating the layers difficult. Increased variability can result as it is difficult to collect the entire organic layer. This is also a labor-intensive technique (16). LLE can also be used to partition interfering materials into the organic layer, while the aqueous layer is collected for analysis.
SLE is analogous to LLE but with significant workflow advantages. Aqueous samples are loaded onto the sorbent (commonly refined, modified diatomaceous earth), and a water-immiscible solvent is added to elute the analytes of interest. The aqueous layer remains absorbed on the sorbent while the organic solvent flows through. No emulsions are present, and SLE can easily be automated. Like LLE, this technique works best when analytes have a log P >1 and are neutral (17). The aqueous layer cannot be collected, as it remains absorbed on the SLE material.
Solid-Phase Extraction (SPE)
SPE methods are the most selective and yield the cleanest extracts. However, they are the most expensive and labor-intensive. A typical method includes conditioning and equilibrating the sorbent, usually with an organic solvent, followed by an aqueous buffer, to prepare the sorbent to receive the aqueous sample. Next, a pretreated sample is loaded, followed by wash steps. An aqueous wash removes water-soluble interferences. A second wash using an organic solvent or an aqueous-organic solvent solution (for example, 50:50 water:methanol or water:acetonitrile) can be added to remove more non-polar interferences. Controlling the pH and optimizing solvent strength ensures the analytes of interest remain bound to the sorbent while interferences are removed. Lastly, compounds are eluted from the sorbent, and the eluent is usually evaporated and reconstituted before analysis. Sorbents can be silica or polymer-based. Polystyrene-divinylbenzene (PSDVB) polymeric sorbents are stable across the entire pH range. Many manufacturers modify the hydrophobic polymer with hydrophilic functional groups to make it water-wettable, so the conditioning and equilibration steps can be eliminated.
Hydrophobic interaction (reversed-phase) SPE methods rely on the partitioning of the analytes between the hydrophobic sorbent and extraction solvent for retention and elution, similar to reversed-phase LC separations. The non-polar interactions are not as strong as ionic retention mechanisms, so the strength of any wash step containing an organic solvent must be carefully controlled, so that target analytes are not eluted during sample clean-up. Washes with only 5–10% organic solvent are common for more hydrophilic analytes. Stronger organic wash solutions can be used for more hydrophobic compounds. This technique can be used for non-ionized, hydrophobic analytes like steroids, or acidic and basic analytes at a pH where they are neutral. Very hydrophobic charged compounds may also be extracted.
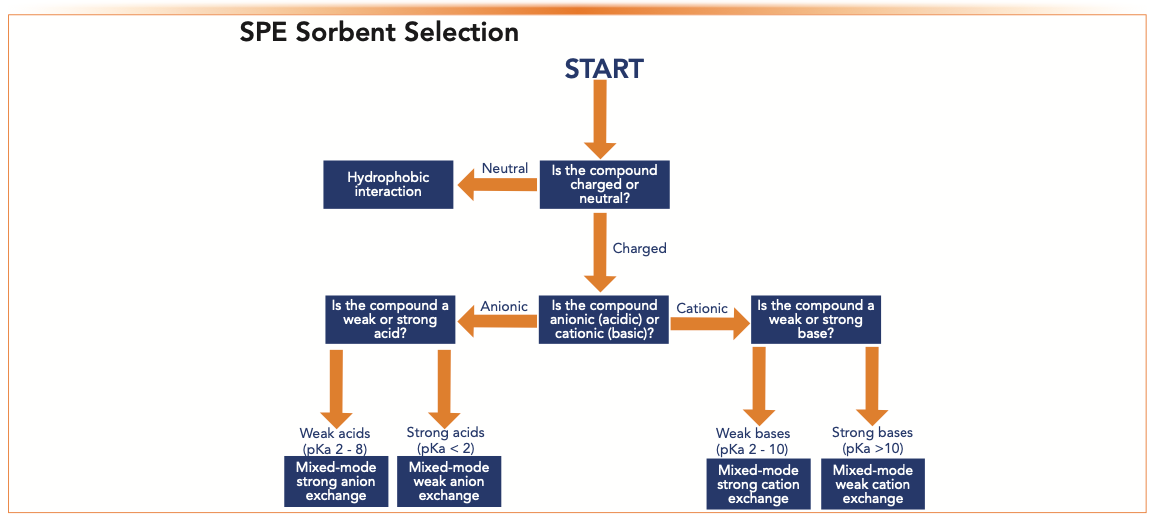
Mixed-mode ion-exchange SPE products are reversed-phase sorbents modified with an ionic functional group. Analytes are retained by nonpolar and ion-exchange interactions. Ion-exchange is a stronger retention mechanism than hydrophobic interaction, so a stronger organic wash can be used to produce cleaner extracts for ionized compounds. Strong cation- or anion-exchange sorbents have a strong acid or base (such as sulfonic acid or quaternary ammonium) as functional groups. These are charged over the entire pH range. These mixed-mode sorbents work well for weak bases or weak acids, respectively. Analytes of interest should be charged during sample load by pretreating the sample using the pH rule of 2 described previously. The charge is then “turned off” during elution. Retention and elution are controlled by modifying the pH of the sample. Weak cation- or anion-exchange chemistries have a weak acid or base as functional groups (carboxylic acid or primary/ secondary amine). They are used to extract strong bases or strong acids, respectively. Strong acids and bases are charged across the entire pH range in solution, so retention and elution are controlled by modifying the pH of the sorbent. The sorbent is charged during load and neutral during elution (18). Figure 2 is a flow chart to help determine which SPE sorbent is best based on whether an analyte is acidic, basic, or neutral.
FIGURE 2: SPE sorbent selection chart based on analyte properties.
Automation and Robotics
The ultimate goal of most bioanalytical assays is the delivery of accurate and reliable quantitative results. While no two samples are exactly alike, optimized sample preparation procedures can minimize sample variability and produce cleaner extracts for more robust bioanalysis assays. Given the inherent variability with manual sample preparation procedures compounded by operator skill level, many laboratories have embraced automation and robotics to enhance laboratory productivity, reduce labor costs, and increase assay reliability.
Over the last twenty years, advancements in technology have resulted in the development of reliable automated instruments for the bioanalytical laboratory. Automation has quickly become the foundation of the modern laboratory, including highly customizable multifunctional liquid handlers to smaller task-focused products. Automated workflows translate to less manual intervention, higher reproducibility, and labor savings.
Robotic platforms with multifunctional, fully automated liquid handling abilities are prevalent in larger, high-throughput laboratories with heavy workloads. They are highly customizable for simple transfer steps, and may be programmed for entirely hands-free sample preparation procedures. Most are fitted with multichannel pipetting arms, which are adaptable to both individual test tube workflows and high-throughput assays in 96- or 384-well-plates. Table II describes some common automation platforms categorized by instrument, complexity, functionality, and vendor. The Fluent platform by Tecan and the Biomek i-Series systems from Beckman Coulter are some of the more complex yet versatile instruments on the market today. Integration with an end-user database or laboratory information management system (LIMS) allows these devices to provide added benefits beyond just sample handling. For example, analytical results can be linked to sample handling procedures, providing a clear, reliable chain of custody. This is important in regulated laboratories, or when bioanalytical testing bears legal implications.
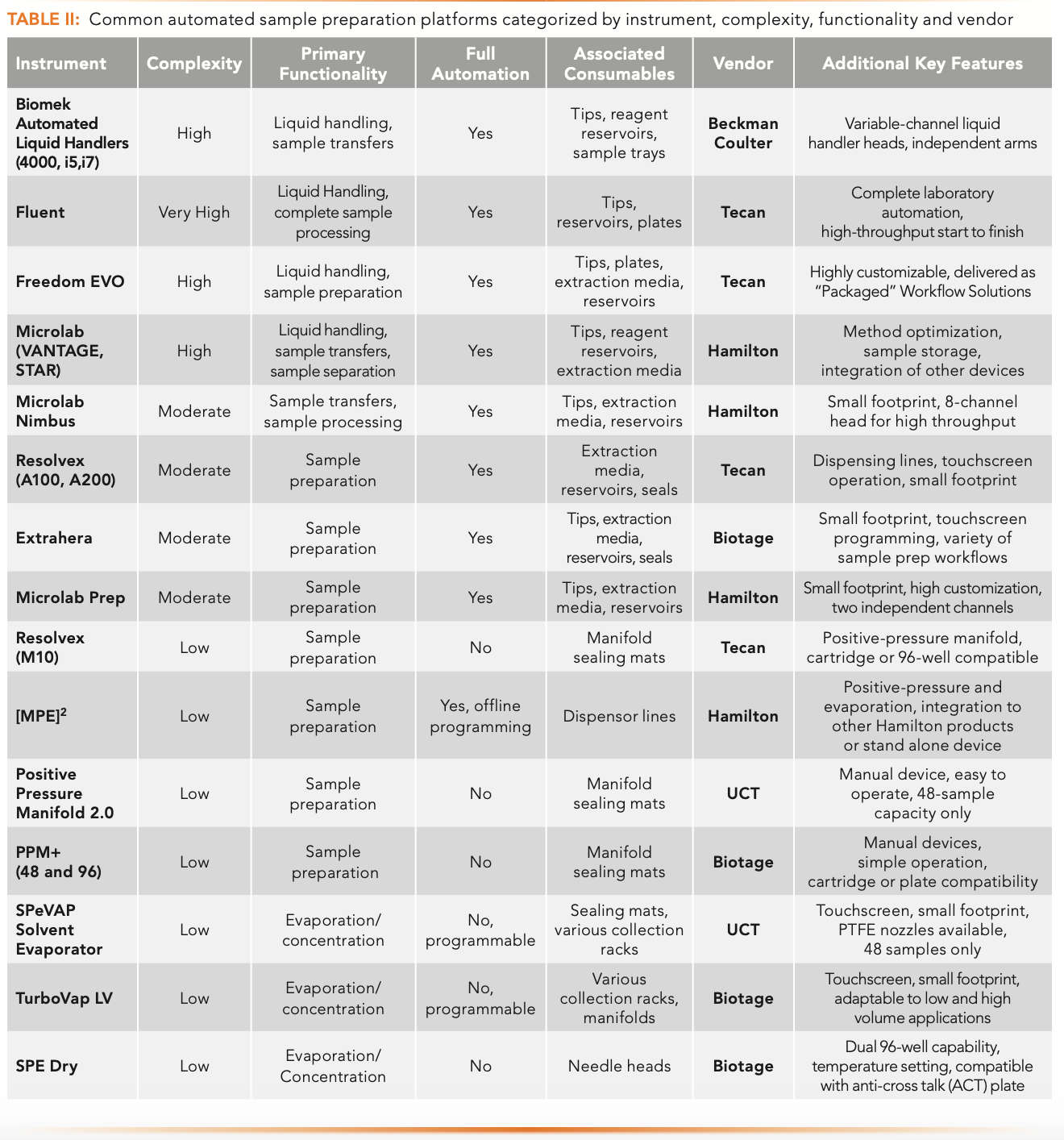
Table II lists common sample preparation automation platforms by instrument, complexity, functionality, and vendor. Fully automated systems provide high reproducibility and limited analyst intervention, but these conveniences do carry a considerable price tag. Besides the initial capital expense, these devices may require the use of vendor-specific consumables, specialized training to operate, and costly service contracts to maintain the investment. Another practical concern with fully automated platforms is the high reliance on a single all-in-one instrument. This model can produce considerable pinch points should the instrument fail or require extensive maintenance. As a result, many laboratories find it necessary to purchase duplicate systems to avoid significant delays in productivity. However, a simpler alternative to this issue is to acquire smaller task-focused robotic devices.
Sample preparation is a challenging step of any bioanalytical workflow. Variability and imprecision may significantly influence the quality of results. Smaller devices of moderate complexity also exist in addition to fully automated robotic platforms. These task-focused platforms enhance reproducibility, minimize manual interventions, and provide high-throughput solutions. These devices typically employ an adaptable stage suitable for column or plate-based extractions, a positive pressure manifold, and either a multichannel pipetting arm or solvent dispensing line (see Table II). The Biotage Extrahera is an automated sample preparation instrument which uses a positive pressure manifold and an eight-channel pipetting arm to execute many different extractions. These include filtration protocols, pass-through SPE, SLE, and robust traditional SPE methods. Tecan also offers a solution for standalone sample preparation with the Resolvex A200. This instrument offers positive pressure processing with a multi-solvent dispensing line and is suitable for 96-well plates or 1 mL narrow-bore columns. Manual manipulation may be required for initial sample transfers, evaporation, or post-extraction concentration. These platforms focus on the sensitive extraction steps, hence the task-focused nature of their operation. Unlike the all-in-one devices, these units are typically available at a fraction of the cost to purchase, operate, and maintain. They are also more user-friendly to program. They possess significantly smaller footprints aimed at conserving laboratory space. When cost becomes a driving factor, many laboratories further reduce the role of automated robotics to supplement some core functionalities with manual devices.
Features included on automated devices of both high and moderate complexity exist as standalone products from multiple vendors (refer to Table II). Multichannel pipets are available, with manual or semi-automated functionality for initial sample transfers or the addition of buffers, reagents, and solvents during the sample extraction. Positive pressure or vacuum manifolds can accommodate both cartridge and plate formats. The Positive Pressure Manifold 2.0 from UCT is one of several manual devices on the market. This unit is a 48-position device that can accommodate 1, 3, and 6 mL extraction cartridges. Manual concentration systems are available for both plates and cartridges, requiring only an electrical outlet and a source of clean nitrogen for operation. The Biotage TurboVap LV is a small but efficient sample evaporator, accommodating various standard test tubes and vials and offer programmable methods for semi-automated operation.
Modern Trends for Sample Preparation in Bioanalysis
Bioanalytical assay development has evolved with the development of new chemistries and polymeric SPE sorbents. While traditional sample types and extraction methods exist, novel products and approaches are being introduced to streamline and enhance routine analysis. Some new products focus on handling complex chemical extractions, while others aim to simplify analyses using low specimen volumes. Bioanalytical assays are trending towards less invasive collection devices, and alternate matrices, like tissue extracts.
The most common and traditional technology for the clean-up of bioanalytical samples involves chemically modified silica products. Common issues with these products include channeling in the silica bed, premature drying, and moderate retention. In addition, bonded silica phases are often not stable at high and low pH, running the risk of hydrolysis of the bonded phase at low pH and dissolution of the silica backbone at high pH. The solution has been the introduction of mixed-mode polymer extraction chemistries. Most of these products are manufactured using a PSDVB backbone, which may be modified with various functional groups to facilitate ion-exchange and hydrophobic interactions. As a result, the retention mechanism is typically stronger than silica particles, and the polymer backbone is more resistant to channeling and premature drying during extraction procedures. This translates to simpler and easily automated extractions, capable of delivering rapid and cleaner eluates for analysis.
Limited specimen volume is a commonly encountered issue for many bioanalytical assays. For example, clinical neonatal tests for acylcarnitine may be supplied with as little as 50 to 100 μL of the sample, because collecting specimens from newborns is a delicate task (19). Additionally, some laboratories may need to perform several different types of analysis on a single sample, such as serum for steroids, xenobiotics, and electrolytes. In many cases, specimen volumes are limited. High purity extraction solvents are expensive, so minimizing the waste of these materials is paramount. One trending solution to these problems is the use of micro-elution SPE products. The first option is loosely packed sorbents with selective chemistries fitted into narrow but hollow plastic tips. The typical bed volume is 0.5 to 5 mg. These are then fitted into a 96-well-plate for high throughput workflows. The resulting extraction protocol requires lower specimen volume and reduced solvent use, typically 50–100 μL for washes, and 30–50 μL for elution. Eluates can be concentrated for increased sensitivity or diluted, eliminating the evaporation step.
A second micro-elution format uses a dispersive pipet extraction (DPX), a technology approach consisting of loosely packed SPE resins inside individual pipet tips. These tips may be fitted to manual pipets or fully automated liquid handlers. The extraction process involves introducing samples, retaining analytes, and removing interferences by multiple aspirate-and-dispense steps to utilize a dispersive SPE technique. Compounds of interest are eluted with conventional solvents. This approach is highly adaptable and yields reasonable recoveries (20), yet samples with higher viscosities, such as blood and tissue, may prove challenging to manage with dispersive pipet extractions.
Solid-phase microextraction (SPME) uses a fiber-coated frit incorporating an SPE extraction phase with an affinity for the analytes of interest. Here, exposing the fiber to liquified samples results in a partitioning of analytes from the sample matrix to the extraction phase. The extraction requires no solvent, and analytes are concentrated on the fiber. The fiber can be placed in a small amount of solvent for desorption and LC–MS analysis. This technology is ideal for gaseous, liquid, and solid samples stemming from field-based applications, such as environmental studies. Applications of SPME in high-throughput bioanalysis with moderate recovery and reproducible data have been published (21).
In addition to novel chemistries and products, the use of alternate matrices and collection devices have altered approaches to bioanalytical method development. One highly intriguing approach involves bloodspot analysis. Small drops of blood may be collected onto thin, paper-like sampling devices with a mere prick of the finger. Once at the laboratory, these blood spots may be subjected to further chemical extractions, digestions, or washes targeting the analysis of numerous xenobiotics or endogenous biomolecules. Volumetric absorptive microsampling (VAMS) is a novel approach that permits collecting a fixed volume of whole blood using a sampling device with a porous hydrophilic tip to collect small, accurate, and precise blood volumes. The blood in the device is dried for storage or transport. These samples can be extracted with an appropriate solvent, which can undergo further sample clean-up by LLE, SLE, or SPE (22).
The advancement of technology improves the quality of bioanalytical analysis while reshaping applications by introducing new products and novel sampling techniques. Choosing an automated platform is no simple task. Throughput needs, budgetary restrictions, and LIMS compatibility are the primary considerations. Regardless of the workflow design, an automated or manual solution is available for sample preparation to ensure accurate and reproducible results.
Method Development Case Study for a Weakly Basic Drug in Plasma
Sample preparation method development begins by researching analyte properties. Our hypothetical drug is a weak base with a primary amine. A deuterated internal standard (IS) is available. Using calculated values from Chemicalize.com, the molecule has a log P of 2.1 and a pKa of 8.2. The desired LOQ is 10 ng/mL, and the desired plasma volume is 200 μL. Once our LC–MS/MS method is optimized and sensitivity is established, we can begin our extraction experiments.
Based on the matrix and analyte properties, sample preparation options are phospholipid depletion/protein removal (because the matrix is plasma), LLE or SLE (since the analyte is hydrophobic), and mixed-mode strong cation-exchange SPE (as the analyte is a weak base). Sample preparation method development should not be done at the LOD or LOQ or using a single data point. Instead, spiked samples should be prepared and extracted in duplicate or triplicate at a single concentration 5 to 10 times the LOQ, in this case, 50 to 100 ng/mL. Include an extraction blank (EB, reagents with no specimen) and negative control (NC, analyte-free matrix with only IS added). Averaged area counts from triplicate extracts are compared to a no matrix, “unextracted” standard (UNX, analyte, and internal standard in reconstitution solvent) at the same concentration as the extracted sample to calculate the process efficiency. Once the best sample preparation method is determined, linearity, external QC, positive and negative specimens, recovery, matrix effect, and process efficiency (23) are evaluated using the final, optimized method conditions.
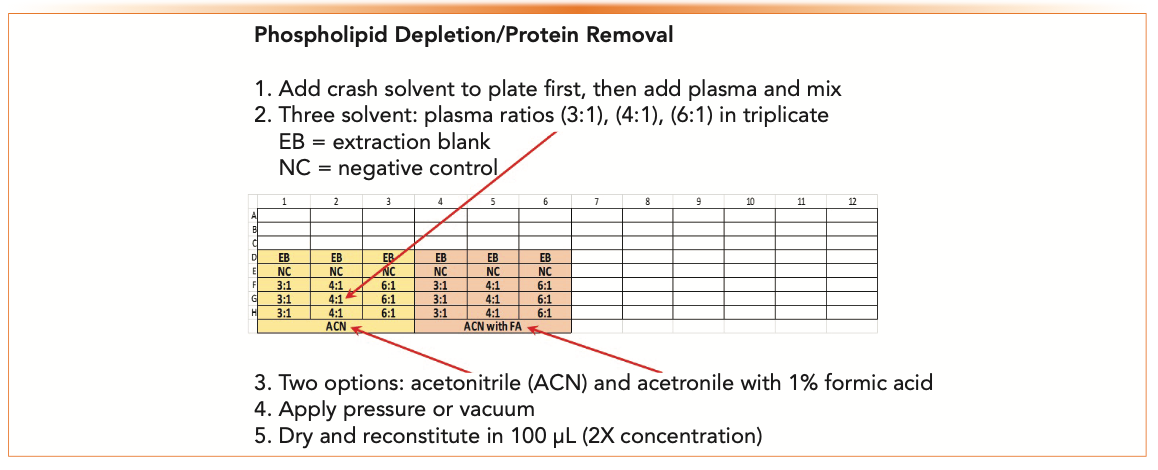
Phospholipid depletion involves minimal method development. Different ratios of the specimen to crash solvent can be scouted. Some products recommend the addition of an acid to increase the recovery of more polar analytes. Acetonitrile usually provides cleaner extracts than methanol, but either solvent can be used. Figure 3 shows an example of a 96-well plate (a “plate map”) configured to evaluate different conditions for phospholipid depletion using acetonitrile. Variables are three different crash solvent to plasma ratios (3:1, 4:1, and 6:1) and the addition of 1% formic acid to the crash solvent. Samples are extracted and analyzed in triplicate at 50 ng/mL (5x the desired LOQ) with an EB, NC, and UNX.
FIGURE 3: A 96-well plate configured to evaluate phospholipid depletion and protein removal SPE for a hypothetical weakly basic drug in plasma.
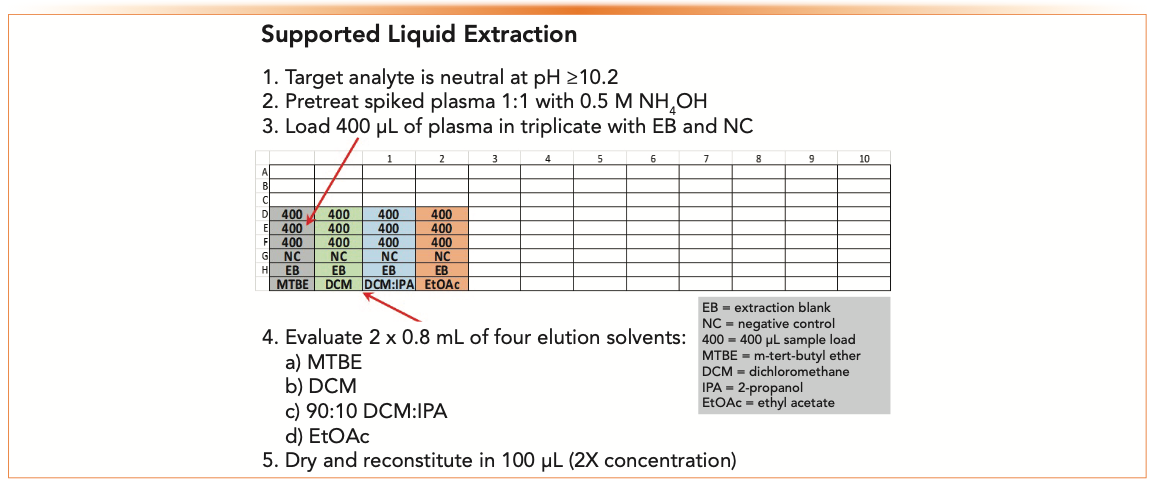
LLE or SLE works best if the analyte is neutral. The primary amine would be neutral 2 units above its pKa (pH 10.2 or higher). Pretreating plasma 1:1 with 0.5 M ammonium hydroxide will neutralize the primary amine for partitioning into the water-immiscible solvent. Figure 4 shows a plate map to scout four different elution solvents using SLE. Spiked samples at 50 ng/mL are analyzed in triplicate with an EB, NC, and UNX.
FIGURE 4: Plate map to evaluate four different elution solvents using supported liquid extraction (SLE) to extract a weakly basic drug in plasma. Load and elution volumes are dependent upon SLE capacity. This experiment uses a 400 μL SLE+ plate.
The analyte must be positively charged for polymeric mixed-mode strong cation-exchange SPE. The weakly basic drug would be charged at a pH <6.2. Pretreating plasma 1:1 with 0.1 M pH 5 ammonium acetate buffer or 2% formic acid will accomplish this. However, 2% formic acid will also disrupt plasma proteins if protein binding is a concern.
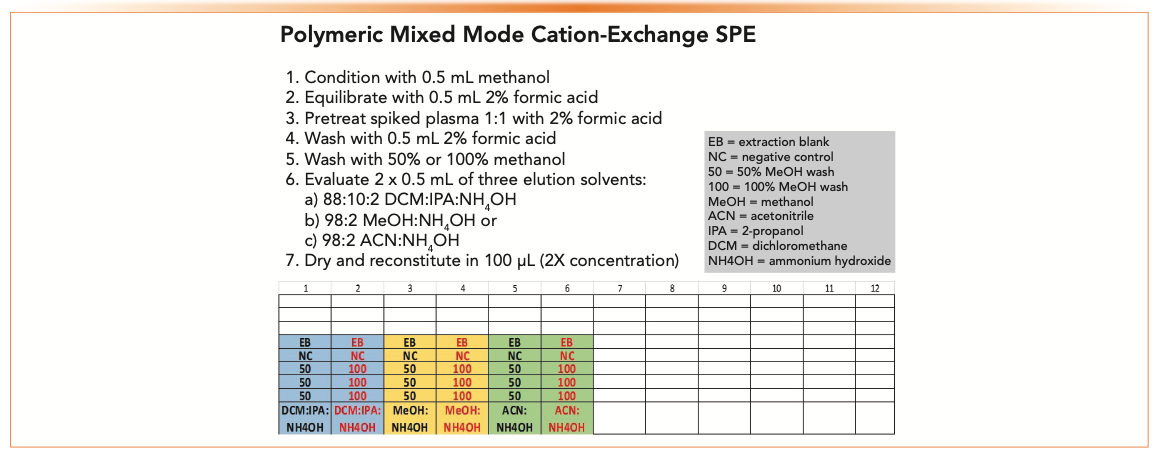
Figure 5 shows a plate map for triplicate samples pretreated with 2% formic acid. Our analyte should be retained by cation exchange which means a 100% methanol or acetonitrile wash will provide the best cleanup. In this case, we will scout a 50% methanol and a 100% methanol wash and three different elution solvents: 88:10:2 dichloromethane: 2-propanol: ammonium hydroxide (DCM:IPA:NH4OH), 98:2 methanol:NH4OH, or 98:2 acetonitrile:NH4OH.
FIGURE 5: Plate map to scout two organic washes and three elution solvents to extract a weakly basic drug in plasma using polymeric mixed-mode strong cation-exchange SPE. Volumes are dependent upon sorbent bed mass. This experiment is set up for a 10 mg plate.
The load and wash steps are collected to analyze for troubleshooting if low or no recovery of the target analyte is observed. If the analyte is in the load step, it was never retained. If the analyte is in a wash step, the wash was too aggressive and became an elution solvent. If it is not in the load, wash, or elution fractions, it was retained on the sorbent and never eluted, possibly decomposed, evaporated or was “stuck” to the elution vial or collection plate due to non-specific binding.
Elution can be done with a single fraction of solvent or two smaller aliquots. Improved recovery and better precision are usually seen with two fractions of elution solvent (2 x 0.5 mL) compared to a single application (1 mL).
Scouting sample preparation options using plate maps can generate simple experiments that investigate the effects of a few variables, or use more of the plate to evaluate more options. The plate map in Figure 4, for example, could be modified to evaluate the ammonium acetate pretreatment, an additional wash (like acetonitrile in addition to methanol), or additional elution solvents.
Process efficiencies for each sample preparation option are reviewed, and the best option is selected for further development. Calibrators, patient specimens, casework samples, proficiency testing samples, or other external QC are analyzed, and then recovery, matrix effect, and process efficiency are determined prior to full method validation (23).
Developing a new extraction method can seem daunting at first. However, knowing about the compounds in the panel, the matrix, the specifics about the extraction technique, and the use of plate maps can make the method development process faster and more efficient.
Summary and Conclusions
This article provided an overview of sample preparation fundamentals for the bioanalysis of physiological samples for the novice and updated guidance in selecting media and the best operating practices for the experienced practitioner. Modern trends summarizing newer SPE media, approaches, and automation platforms were discussed. A case study for sample preparation method development for a weak base in plasma and the optimization process demonstrated the recommended procedures for using various sample preparation approaches.
Acknowledgments
The authors would like to thank the following colleagues for the timely review of the manuscript to improve its accuracy and clarity: Stephen Merrigan, MS, R&D Scientist, ARUP Institute for Clinical and Experimental Pathology, Doug Raynie, Ph.D., Department Head, and Associate Professor, South Dakota State University, Jinlan Dong, Ph.D., Bioanalytical Research Scientist, Corteva Agriscience, Naidong Weng, Ph.D., Scientific Director and Head of Bioanalytical and Pharmacokinetics US East Coast, Discovery Sciences, Janssen and Johnson and Johnson, and Perry Weng, Ph.D., Research Chemist, USFDA.
References
(1) S. Moldoveanu and V. David, Modern Sample Preparation for Chromatography (Elsevier, Amsterdam, the Netherlands, 2nd Ed., 2021).
(2) M.W. Dong, HPLC and UHPLC for Practicing Scientists (Wiley, Hoboken, New Jersey, 2nd Ed., 2019).
(3) S. Ahuja and M.W. Dong, Eds., Handbook of Pharmaceutical Analysis by HPLC (Elsevier, Amsterdam, the Netherlands, 2005).
(4) W. Li, W. Jian, et al. Sample Preparation in LC-MS Bioanalysis (Wiley, Hoboken, New Jersey, 2019).
(5) D.E. Raynie, LCGC North Am. Columns in 2015–2021.
(6) Blood Basics, https://www.hematology.org/education/patients/blood-basics#:~:text=It%20has%20four%20 main%20components,to%20prevent%20 excess%20blood%20loss (accessed April 26, 2021)
(7) Urine Composition and Function, https://chem.libretexts.org/@go/page/86580, (2020, accessed April 26, 2021)
(8) https://homogenizers.net/pages/ac-tissue-homogenization (accessed April 26, 2021).
(9) S. K. Bhal, “Lipophilicity Descriptors: Understanding When to Use Log P & Log D,” Advanced Chemistry Development, Inc., Toronto, ON, Canada. https://www. acdlabs.com/download/app/physchem/logp_vs_logd.pdf (accessed April 26, 2021).
(10) A. C. Lee and G. M. Crippen, J. Chem. Inf. Model. 9, 2013–2033 (2009). https://doi.org/10.1021/ci900209w.
(11) https://chemicalize.com/welcome (accessed April 26, 2021).
(12) https://hmdb.ca/ (accessed April 26, 2021).
(13) R.C. Baselt, Disposition of Toxic Drugs and Chemicals in Man (Biomedical Publications, Seal Beach, California, 12th Ed., 2020).
(14) J. Stone, “Sample preparation techniques for mass spectrometry in the clinical laboratory,” in Mass Spectrometry for the Clinical Laboratory (MSACL, 2017).
(15) R. Perestrelo, et al., Analytica Chimica Acta 1070, 1–28 (2019). https://doi.org/10.1016/j.aca.2019.02.036
(16) Liquid-Liquid Extraction. https://chem.libretexts.org/@go/page/2024 (accessed April 26, 2021.
(17) L. Silvestro and S. R. Savu, Bioanalysis 7, 17 (2015). https://doi.org/10.4155/bio.15.144.
(18) B.U.W. Lei and T.W. Prow, Biomed. Microdevices 21, 81 (2019).
(19) M.l.J. Telepchak, T.F. August, and G. Chaney, Forensic and Clinical Applications of Solid-Phase Extraction (Humana Press, Totowa, New Jersey, 2004).
(20) S.T. Ellison, W.E. Brewer, and S.L. Morgan, J. Anal. Tox. 33, 356–365 (2009).
(21) M. Maciążek-Jurczyk, V. Bessonneau, J. Ings, L. Bragg, M. McMaster, M. R. Servos, B. Bojko, and J. Pawliszyn, Anal. Bioanal. Chem. 412, 4183–4194 (2020).
(22) P.E. Minkler, M.S.K. Stoll, S.T. Ingalls, S. Yang, J. Kerner, and C.L. Hoppel, Clin. Chem. 54, 1451–1462 (2008).
(23) B.K. Matuszewski, M.L. Constanzer, and C.M. Chavez-Eng, Anal. Chem. 75, 3019–3030 (2003).
ABOUT THE COLUMN EDITOR
Michael W. Dong is a principal of MWD Consulting, which provides training and consulting services in HPLC and UHPLC, method improvement, pharmaceutical analysis, and drug quality. He was formerly a Senior Scientist at Genentech, a Research Fellow at Purdue Pharma, and a Senior Staff Scientist at Applied Biosystems/PerkinElmer. He holds a PhD in Analytical Chemistry from City University of New York. He has more than 130 publications and a best-selling book in chromatography. He is an editorial advisory board member of LCGC North America and the Chinese American Chromatography Association. Direct correspondence to: LCGCedit@mmhgroup.com.

ABOUT THE AUTHORS
Stephanie J. Marin is a Senior Applications Chemist at Biotage in Salt Lake City, UT. She received her PhD in chemistry from Arizona State University and has expertise in sample preparation, LC-MS, and the development and validation of clinical assays. She is the author of over 30 peer-reviewed publications and book chapters.

Jeremy Smith is an Applications Chemist supporting the Analytical Life Sciences markets at Biotage in Charlotte, NC. He holds a BS in Biology from the University of Kentucky.

Jillian Neifeld is an applications chemist at Biotage in Fairfield, CA. She has a MS from Virginia Commonwealth University.

Elena Gairloch is a Science Support Manager at Biotage in Bellefonte, PA. She has a BS in Chemistry and Biology from Lynchburg College and has worked in the HPLC/Sample Prep industry for over 30 years, innovating in the Separation Sciences.


New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.














