Automating Analysis of Antibiotics in Blood
Therapeutic drug monitoring (TDM) is an important method for determining both dose and optimal effective level of a drug in the blood, preventing side effects such as kidney damage. Using the antibiotic classes aminoglycoside and vancomycin as examples, an analysis method is presented that combines fully automated sample preparation with ultrahigh-pressure liquid chromatography–triple quadrupole-mass spectrometry (UHPLC–TQMS).
DIgilife/stock.adobe.com
Therapeutic drug monitoring (TDM) is an important method for determining both dose and optimal effective level of a drug in the blood, preventing side effects such as kidney damage. Using the antibiotic classes aminoglycoside and vancomycin as examples, an analysis method is presented that combines fully automated sample preparation with ultrahigh-pressure liquid chromatography–triple quadrupole-mass spectrometry (UHPLC–TQMS).
Aminoglycoside antibiotics and vancomycin are used to treat severe infections, especially gram-negative enterobacteria. Multidrugâresistant germs often lead to life-threatening issues, such as respiratory tract infections, urinary tract infections, bloodstream (sepsis), or post-surgical (wound) infection (1,2). Infections caused by multiâresistant Pseudomonas or Acinetobacter germs are especially problematic in intensive care units. In the context of infections, resistance to classical antibiotics, such as beta-lactams or (fluoric) quinolones, is being increasingly observed (3). This translates into a longer stay for patients, a higher morbidity, and a higher mortality rate.
As a result of the shortage and declining development of new antibiotic classes (4), combined therapy with aminoglycosides and vancomycin is increasingly being used to treat such infections. These are classes of antibiotics that were used in the past to treat resistant bacteria, but which have been phased out over the past 20 years because of their toxicological risks and the historically increasing importance of beta-lactams.
Combined LC and MS Method for Therapeutic Drug Monitoring
Today, aminoglycosides are among the few effective preparations for the treatment of serious bacterial infections because of growing resistance Aminoglycosides and vancomycin have a limited therapeutic range. In high dosages, the products are harmful to the kidneys, meaning that a patient’s blood level must be adjusted precisely and monitored for effective dosage. This soâcalled therapeutic drug monitoring (TDM) has become wellâestablished in recent years, but the immunoassays used as standard can lead to false results caused by cross-reactivity. Analytes with a similar chemical structure cannot be measured reproducibly with this method.
An increasing number of processes using modern liquid chromatography (LC) coupled with mass spectrometric (MS) analysis have therefore been established as the gold standard for TDM. LC–MS/MS triple quadrupole analysis with its substance selectivity, high sensitivity, robustness of the system, and comparatively low cost per analysis has become the method of choice. However, tedious sample preparation has been limiting the use of LC–MS/MS in the clinical environment. An important development is coupling LC–MS/MS systems with automated sample preparation systems.
An automated LC–MS/MS method for the simultaneous analysis of seven aminoglycosides and vancomycin is described below.
Materials and Methods
Reagents: The analytical standards used were the antibiotics amikacin, gentamicin (mixture of C1, C1a, and C2/C2a), kanamycin, neomycin B, paromomycin, tobramycin, and vancomycin from Sigma Aldrich, and hygromycin B from Wako Chemicals. Individual stock solutions of 100 mg/mL concentration were prepared in water and diluted with blank plasma to prepare the calibration standards (seven concentration levels) and control samples (quality control [QC], four levels). The calibration range was between 0.1 and 50 µg/mL (0.1 and 100 µg/mL for vancomycin).
Since no arbekacin analytical standard is currently available on the market, a fiveâlevel + blank plasma calibration set from Microgenics Corporation was used. The highest calibration level was additionally diluted to extend the limit of the quantification range, which was then set at 0.1 to 30 µg/mL. All other reagents used by Sigma-Aldrich were of analytical quality. The LC–MS solvents were supplied by Wako Chemicals.
Sample Preparation: Automated sample preparation was performed with the CLAM-2030 sample preparation module coupled directly to the LC–MS/MS system (Shimadzu). All calibration standards, controls, and samples were analyzed using the same procedure. The filters of the sample preparation were activated with 20 µL of a mixture of 1:3 water–isopropanol, and 100 µL of the precipitating reagent (trichloroacetic acid 100 g/L in water) was added. Subsequently, 20 µL of the internal standard (hygromycin B, 50 µg/mL in water) and 20 µL of the plasma sample were introduced. The samples were then mixed for 1 min and the precipitate filtered for 90 s. The extract was then transferred by the sample preparation system to the autosampler of the LC–MS/MS system. In the sample preparation process, the individual steps were nested to increase sample throughput accordingly.
Measurement parameters are described below.
LC (HILIC) Conditions: System: Nexera X2 (Shimadzu); LC column: 50 × 2.1 mm, 3-μm InertSustain Amide (GL Sciences); temperature: 50 °C; mobile phase: A: water 250 mM ammonium formate 1% formic acid, B: acetonitrile; flow rate: 400 μL/min; injection volume: 0.5 µL; gradient: 75% B (0.2 min) to 55% B (in 1.3 min) - 55% B (for 1.5 min) to 75% B (in 0.1 min); total run time: 4.75 min.
MS/MS Conditions: System: LCMS-8060 (Shimadzu); ionization: heated ESI; ESI voltage: + 5 kV; temperature: interface: 300 °C, desolvation line: 150 °C, heater block: 500 °C; gas flow: atomizing gas: 2.5 L/min, heating gas: 10 L/min, drying gas: 3 L/min; dwell time/pause time: 23 ms/1.5 ms; multiple reaction monitoring (MRM): amikacin: MRM quant., 586.3 > 163.3, MRM qual., 586.3 > 425.2; arbekacin: MRM quant., 553.4 > 163.1, MRM qual., 553.4 > 425.3; gentamicin C1a: MRM quant., 450.4 > 322.1, MRM qual., 450.4 > 163.1; gentamicin C1: MRM quant., 478.4 > 322.2, MRM qual., 478.4 > 157.1; gentamicin C2: MRM quant., 464.4 > 322.1, MRM qual., 464.4 > 160.1; hygromycin: MRM quant., 528.3 > 352.1, MRM qual., 528.3 > 256.9; canamycin: MRM quant., 485.4 > 163.1, MRM qual., 485.4 > 324.0; neomycin B: MRM quant., 615.4 > 163.1, MRM qual., 615.4 > 163.0; paromomycin: MRM quant., 616.4 > 293.1, MRM qual., 616.4 > 163.1; tobramycin: MRM quant., 468.3 > 324.1, MRM qual., 468.3 > 163.1; vancomycin: MRM quant., 725.4 > 1307.2, MRM qual., 725.4 > 144.4.
Results
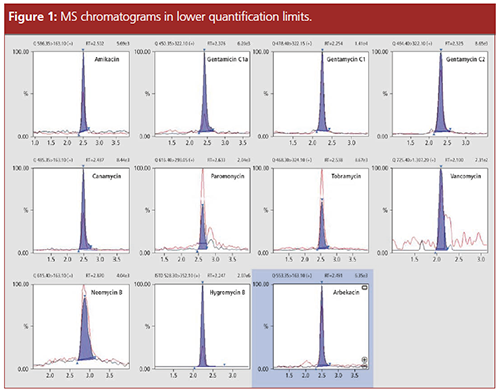
Calibration: Calibration lines were created with internal standardization using quadratic regression (weighting 1/X or 1/X2). For acceptance criteria, accuracy of between 85% and 115% was assumed. Figure 1 shows the chromatograms of the standards in the range of the lower quantification limit.
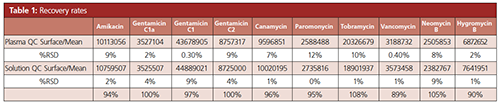
Recovery Rate: The total recovery (combination of extraction and matrix effect) was determined by comparing the signal areas of the mean control samples in plasma to corresponding controls in solvent. The measurements were performed in triplicate. The results of the recovery are summarized in Table 1. Mean recovery rates were between 89% and 105%, demonstrating a good extraction rate with a low matrix effect.
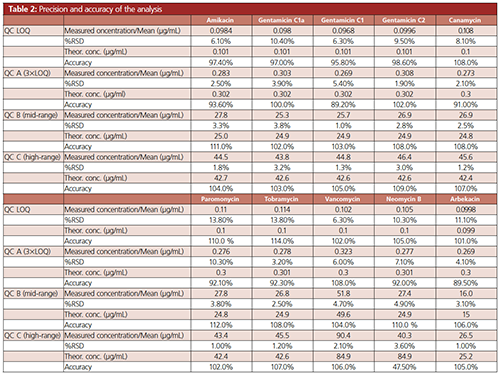
Precision and Accuracy: Precision and accuracy of the measurements were determined by measuring the concentration of the QC samples over four concentration levels in three independent runs (three days). Every day, five replicates of each QC were processed and analyzed. Acceptance criteria were a relative standard deviation <15% (20% at LOQ) and an accuracy between 80–120%. The results are shown in Table 2.
Conclusions
The combination of fully automated sample preparation with triple quadrupole LC–MS/MS is an ideal platform to ease the use of mass spectrometry in the clinical environment and to improve TDM urgency. This article has demonstrated the benefit of using automated sample preparation with LC–MS/MS for automated and multiplexed analysis of aminoglycosides and vancomycin in blood plasma.
The automation, low susceptibility to errors, and simple operation improve the monitoring of therapeutic drugs significantly. Accurate quantification and good recovery rates are indispensable for robust and routine laboratory operation.
References
- G.M. Rossolini, E. Mantengoli, J. Docquier, R.A. Musmanno, and G. Coratza, New Microbiol.30, 332 (2007).
- G. De Angelis, T. D’Inzeo, B. Fiori, T. Spanu, and G. Sganga, J. Med. Microbiol. Diagn.3, 132–8 (2014).
- M. Marchiso, A. Porto, R. Joris, M. Rico, M.R. Baroni, and J. Di Costa, Braz. J. Microbiol.46(4), 1155–1159 (2015).
- A.R.M. Coates, G. Halls, and Y. Hu, Br. J. Pharmacol.163(1), 184–194 (2011).
Stephane Moreau obtained his diploma from INSA in fine chemistry and engineering with a specialization in chemical process engineering in 1994. He then started his professional career in laboratory equipment distribution before he joined the brand new Shimadzu France subsidiary in 2002. Since then, he has held various positions to develop the MS range business. Since September 2013, he has been product manager for the MS range with Shimadzu Europe.

Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.











