Assessing Permeation of VOCs Through Polymeric Materials
The Column
The migration of chemicals through polymeric materials is difficult or impossible to model theoretically, placing an emphasis on experimental assessment to provide reliable empirical data. This article describes an investigation into the permeation of volatile chemicals through thin polymer membranes based on dynamic headspace, and how the information generated may be of value industrially - specifically in the fields of food packaging and personal protective equipment.
Photo Credit: Zora Rossi/Shutterstock.com

Hannah Calder, Caroline Widdowson, and David Barden, Markes International, Llantrisant, Wales, UK.
The migration of chemicals through polymeric materials is difficult or impossible to model theoretically, placing an emphasis on experimental assessment to provide reliable empirical data. This article describes an investigation into the permeation of volatile chemicals through thin polymer membranes based on dynamic headspace, and how the information generated may be of value industrially - specifically in the fields of food packaging and personal protective equipment.
Introduction
Migration of VOCs Through Materials: Understanding the migration of volatile organic compounds (VOCs) through polymer membranes is an important field of study, with two important applications relating to food safety and personal protective equipment.
Applications regarding food largely concern their contamination with exogenous chemicals, either from the environment, or from the packaging material itself (1) because of the potential health concerns. However, the migration of food aroma chemicals through the packaging, and the consequent effect on food quality, is also of interest.
The other main area of research is the efficacy of personal protective equipment (PPE) or chemical protective clothing (CPC), such as the disposable nitrile gloves commonly used in laboratories. Such materials may show wide variations in performance (2), affecting how well they can fulfil their role.
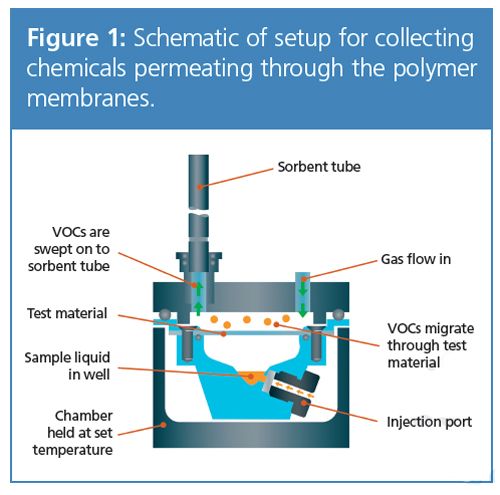
Basic Theory of Migration Through Polymers: The passage of gases and volatile chemicals through thin films of material is wellâunderstood theoretically, and governed by long-established physical laws. In its simplest form, the rate at which a gas or vapour passes through a polymer - its permeability - depends upon three processes: (i) adsorption of the chemical onto the polymer surface; (ii) diffusion through the polymer; and (iii) desorption of the chemical from the polymer (3).
However, these basic processes are complicated by a large number of factors, such as the variation in polymer microstructure, chemical interactions between the volatiles and the polymer, the presence of polymer additives, whether or not a source of volatiles is in direct contact with the material, and environmental variables, such as humidity and temperature. Additionally, the exposure of polymers to chemicals can itself affect their permeability, as well as their mechanical properties.
Such factors make all but the simplest of systems very difficult to model, and means that comprehensive experimental data is needed for reliable characterization. For the analyst, the need for a straightforward approach to obtain such data is made more urgent by the continuing development of advanced coatings and packaging materials - including laminates, polymer blends, bioâbased materials, and nanocomposites.
In this article, we describe the use of an approach that allows permeation of chemical vapours through polymer films or sheets to be assessed. We show how, in conjunction with thermal desorption–gas chromatography–mass spectrometry (TD–GC–MS), this technique can be used to generate empirical data on the permeation of VOCs through three types of polymer films.
Experimental
Materials: Three polymeric materials were investigated: (a) polypropylene (PP) with 70 µm thickness, (b) polyethylene terephthalate (PET) with 23 µm thickness, and (c) laminate comprising PET (23 µm), silicon oxide-coated PET (12 µm), and PP (70 µm).
Permeation Sampling: Instrument: A sixâchamber Micro-Chamber/Thermal Extractor with permeation accessory (Markes International) was used to secure in place a section of polymer, leaving 6 cm2 exposed to a source of VOCs in the well below. Sample volume: 10 µL each of limonene and n-dodecane, injected separately into the well of the permeation accessory. Chamber temperature: 30 °C. Flow rate: 50 mL/min dry nitrogen over the membrane, to sweep the escaping volatiles onto a TD sampling tube. Sampling tubes: Stainless steel, packed with Tenax TA (Markes International), changed every 10 min.
TD: Instrument: TD100-xr (Markes International). Flow path temperature: 180 °C. Focusing trap: General-purpose hydrophobic (Markes International). Preâpurge to split: 30 mL/min (1 min). Primary (tube) desorb: 280 °C for 10 min; trap flow: 30 mL/min; inlet split: 30 mL/min. Pre-trapâfire purge: 1 min at 30 mL/min. Secondary (trap) desorb: Trap low: 10 °C; trap high: 350 °C; heating rate: max; hold time: 3 min. Outlet split: 30 mL/min. TD split: 42:1.
GC: Column: 30 m × 0.25 mm, 0.25âµm DBâ5ms (Agilent Corporation). Flow: Constant flow, 1.5 mL/min. Temperature programme: 50 °C (3 min), then 15 °C/min to 300 °C (5 min). Total run time: 24.67 min.
Quadrupole MS: Ion source: 350 °C. Transfer line: 310 °C. Quadrupole: 150 °C. Mass range: m/z 35–450.
Results and Discussion
Experimental Setup: Among the various chemicals potentially of interest, this study focused on (a) limonene (C10H16, b.p. 176 °C), to represent food or fragrance volatiles that might be released from a food sample wrapped in packaging, and (b) n-dodecane (C12H26, b.p. 216 °C), to represent hazardous chemicals that might migrate through the packaging into a food sample - for example, from a printed label.
In brief, the setup involves the permeation of vapours from a sealed compartment containing a mixture of liquid analytes, through a membrane into a chamber. The escaping volatiles are swept with a flow of gas onto a sorbent-packed tube for analysis by TD–GC–MS (Figure 1), in a similar manner to that previously described for dynamic headspace sampling of volatiles released from bulk materials (4). Assessment of linearity from 10–1000 ng gave R2 values of 0.990 and 0.999 for limonene and n-dodecane, respectively.
Polymer Permeation Tests: For the tests of polymer permeation, limonene and n-dodecane were injected into the well of the permeation accessory as a mixture, so that chemical-specific effects on the polymer’s physical structure affected both data sets equally.
The results (Figure 2) show that for both chemicals, PP is far more permeable than PET and laminate, and that the differences between the two sample chemicals are relatively slight.
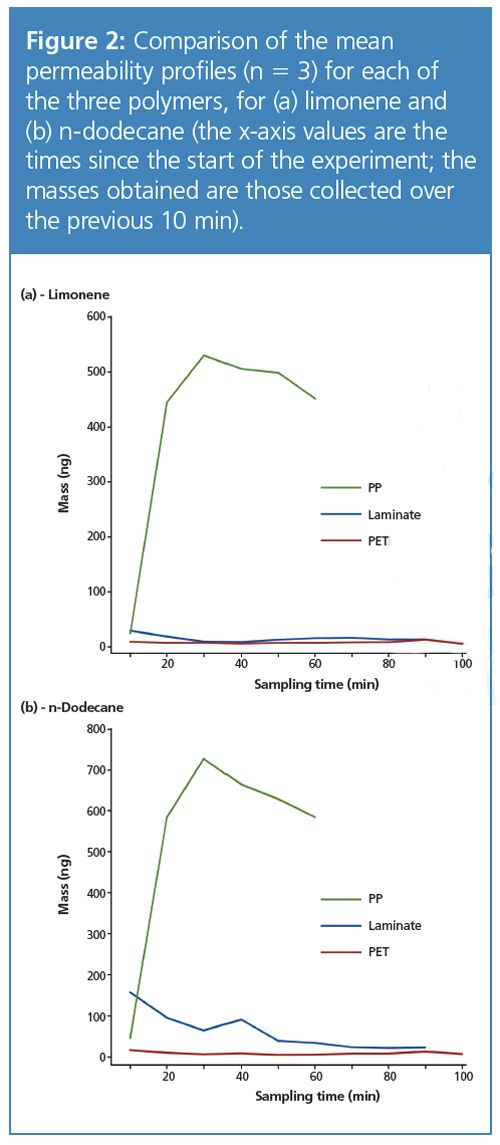
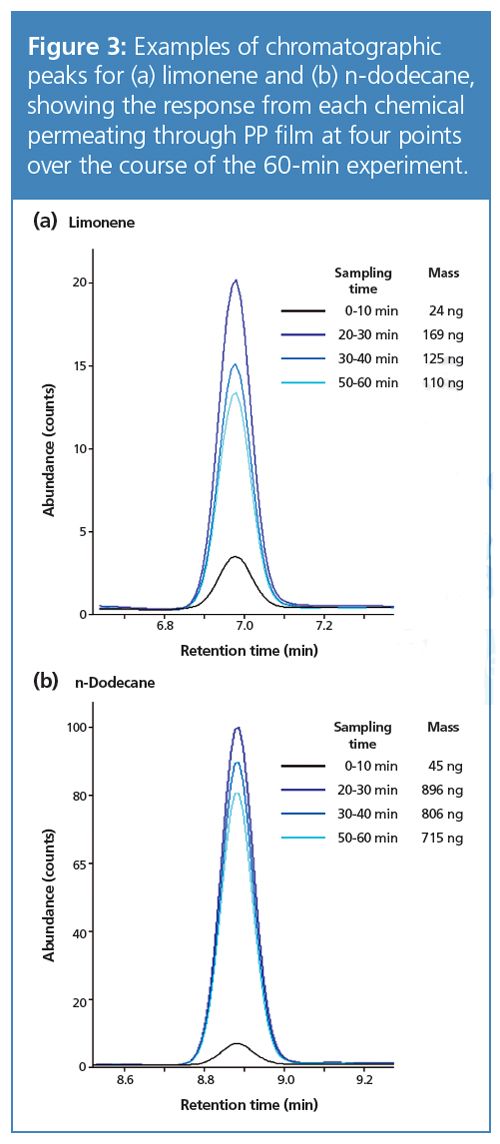
Considering the PP experiment, it can be seen that the amount of limonene and n-dodecane migrating increases sharply over the first 30 min, which is likely a result of the reduction in integrity of the polymer over this time. After this point, the masses migrating through diminish because of the reduction in the vapour concentration as the amount of the two compounds in the well approaches zero. Figure 3 illustrates this progression by two sets of overlaid chromatograms.
PET, on the other hand, shows a comparatively low permeability to both chemicals, and based on this initial assessment would appear to be the most desirable material for reducing chemical permeation.
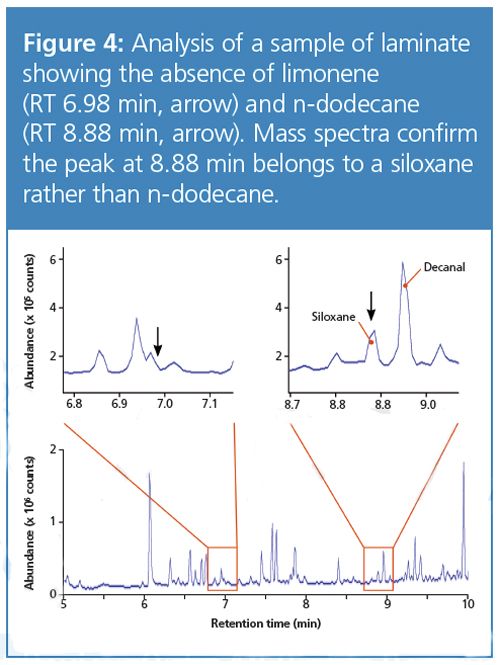
Finally, the laminate sample, which as a composite material might be expected to have a low permeability, actually lets through a considerable quantity of limonene, at a rate that diminishes over the course of the first hour. (To confirm that this was indeed the result of permeation, blank tests showed that the laminate itself did not emit limonene or n-dodecane - see Figure 4). This rather surprising result is an example of the complex properties that can be exhibited by polymeric materials, and one which would perhaps not have been expected theoretically.
Conclusions
In this article, we have shown that a headspace sampler fitted with a permeation device provides a simple and reliable way of investigating the migration of chemicals through membranes - for example, the polymer films used to protect food, or the materials used in personal protective equipment.
Used in conjunction with TD–GC–MS, such an approach can provide data on the rates of migration of chemicals through a material, overcoming the difficulty in predicting how a given material will respond to a particular chemical (or indeed a mixture of chemicals). An added advantage of TD with relevance to this application is the ability to split and re-collect analyte flows, making it easier to validate the analytical method by repeat analysis of one sample.
It is expected that the empirical data generated by this approach will prove particularly valuable when testing barrier materials for food or PPE applications, especially in view of the rising use of polymer composites and other advanced materials that are very challenging to model theoretically.
References
- I.S. Arvanitoyannis and K.V. Kotsanopoulos, Food Bioprocessing Technology7, 21–36 (2014).
- R.N. Phalen and W.K. Wong, Journal of Applied Polymer Science132, 41449 (2015).
- G.L. Robertson, Food Packaging: Principles and Practice (CRC Press, 2013), chapter 4.12, p. 98.
- Markes International Application Notes: 095 – Food decomposition analysis using the Micro-Chamber/Thermal Extractor and TD–GC–MS; 101 – Rapid aroma profiling of cheese using a Micro-Chamber/Thermal Extractor with TD–GC–MS analysis; 104 – Rapid and sensitive aroma profiling of strawberries. Available at www.markes.com/Resources/Application-notes/.
Hannah Calder received an M.Chem. in chemistry from Cardiff University, UK, and her thesis was focused on structural identification and synthesis of organic compounds using a variety of analytical techniques including nuclear magnetic resonance (NMR), X-ray diffraction (XRD), and GC–MS. Hannah joined Markes in 2013 as a technical specialist in the product development department, before moving to sales support, specializing in thermal desorption products and applications.
Caroline Widdowson is the product marketing manager for thermal desorption at Markes International, having completed her chemistry degree at Cardiff University in 2004, followed by a Ph.D. in organic chemistry. As part of her current role, Caroline advises manufacturers, test laboratories, and research institutes on equipment needed to monitor indoor air and study chemical emissions from materials used indoors or in vehicle interiors. Caroline also participates in ASTM and CEN standards and regulatory committees relating to this area.
David Barden is technical copywriter at Markes, having joined the company in 2011. David studied natural sciences at the University of Cambridge, UK, and remained there for his Ph.D. in synthetic organic chemistry, which he received in 2003. A placement at Wiley-VCH in Germany, was then followed by seven years as a journals editor at the Royal Society of Chemistry Publishing, UK.
E-mail:enquiries@markes.com
Website: www.markes.com

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









