Approaches to Singleton Achiral Purification of Difficult Samples for Discovery Research Support
LCGC North America
Several approaches for purifying difficult samples more efficiently for discovery research support are mentioned in this paper. These approaches use mass triggered HPLC on various specialty columns.
Approaches to Singleton Achiral Purification of Difficult Samples for Discovery Research Support in the Pharmaceutical Industry
Chromatographic resolution of pharmaceutical compounds is one of the most efficient approaches for generating small quantities of pure material for biological testing in early pharmaceutical research and development (1–3). The use of chromatographic resolution from a purification group allows more time for synthetic chemists to make more compounds for research. Hence, separation using preparative chromatography rapidly has become a standard approach for the generation of test substances in pharmaceutical research and development in recent years (4,5).

Discovery purification support is a challenging task. Purification scientists often are confronted with large sample loads and short turnaround time. The ability to develop analytical separation methods rapidly and the subsequent ability to scale up separations to preparative columns are critical for meeting sample turnaround timelines. Difficult samples require more time for analytical method development and more effort in handling these compounds on preparative scale. Difficult samples are defined as follows: minimal retention on the regular reversed-phase columns due to high polarity, minimal separation on the reversed-phase columns due to structurally similar impurities such as diastereomers and regioisomers, requirement for large sample amount for numerous injections, and degradation in aqueous solution.
This article addresses three methods for dealing with difficult samples and their challenges. The first method used hydrophilic interaction liquid chromatography (HILIC) stationary phases to increase interaction between the polar functional group of the sample and its columns (6). HILIC columns operate in aqueous normal-phase mode. Therefore, compounds that have no retention on the reversed-phase columns generally have strong retention on the HILIC columns. An inversed gradient of 100% acetonitrile to 50% acetonitrile in water usually was performed on this type of column. As a result, retention of polar ionic compounds increased as the amount of organic solvent in the mobile phase decreased. The separation mode used an eluant containing a water-miscible solvent such as acetonitrile to promote hydrophilic interaction between the analyte and its hydrophilic stationary phase. Based upon the obtained results, HILIC is a technique comparable to traditional normal-phase chromatography and orthogonal to reversed-phase chromatography.
The second approach involved using hydrophilic endcapping columns to retain highly polar compounds. These columns retain polar compounds more efficiently due to a unique hydrophilic endcapping. The combination of a moderately covered hydrophobic group and an additional polar group gives selectivity when compared to a traditional reversed-phase column — yet it allows for more retention of polar compounds. These columns can operate in a highly aqueous solution such as 100% water. Under this condition, some polar compounds can be retained on the column for two or three voids. Under some situations, this retention time should be sufficient for isolating desired components from its crude form.
The third approach pertained to supercritical fluid chromatography (SFC) for achiral purifications. SFC has been proven as a very efficient tool for separation of various compounds (7,8). It is used widely in the pharmaceutical industry to support discovery research. The low viscosity and high diffusivity of supercritical fluid allows the process to work at a higher linear velocity when pass through the column. SFC separations are normally fast and use less organic solvent compared with HPLC. Therefore, SFC is suitable for large-scale separations from grams to kilograms. Gram-scale samples can be time consuming to purify using reversed-phase HPLC–mass spectrometry (MS) columns. SFC not only speeds up the purification process, but also avoids sample degradation in aqueous solution. This is because SFC operates in normal-phase mode. A variety of columns can be used for SFC achiral purifications, including reversed-phase C-18 columns, normal-phase silica gel columns, and pyridine and bonded-phase columns (cyano, diol, and amino). SFC also is used very commonly for chiral separation. Chiral stationary phases also can be used to isolate the structurally similar impurities such as diastereomers and regioisomers.
Difficult samples obtained from Pfizer discovery research presented a challenging task. However, the use of HILIC, hydrophilic endcapping reversed-phase columns, and normal-phase SFC technologies offset these challenges. The following article is a discussion of the analytical method developments, the preparative experimental conditions, and the final purification results obtained from each of the three experiments. The purity and yield of each experiment also are discussed.
Experimental
Material: The compounds purified in this paper were the pharmaceutical substances belonging to and synthesized at Pfizer Global Research and Development (Groton, Connecticut).
The mobile phases used in all studies varied. All were reagent grades from a variety of sources. The stationary phases and columns also were obtained from various sources.
Analytical reversed-phase HPLC:The analytical HPLC system was an LC–MS system from Agilent (Palo Alto, California). This system was used for preliminary analytical screening purposes. The six reversed-phase HPLC columns were Luna C-18, Phenylhexyl, Polar RP, Bonus RP, PFP, and Gemini (Phenomenex, Torrance, California) in either acidic or basic condition. The flow rate in each column was 1.5 mL/min. A general linear gradient from 5% acetonitrile to 95% acetonitrile in water was performed on these columns. If the compound had little or no retention on these columns, the compound was then forwarded to specialty columns (HILIC columns, type-C silica columns, or hydrophilic endcapping columns) for further method development. If the sample was larger in scale or showed very tight separation, SFC methods were then used to purify the compounds.
Preparative reversed-phase HPLC systems: The preparative HPLC systems were HPLC and MS systems from Shimadzu (Columbia, Maryland) or from Waters (Milford, Massachusetts). In this study, the preparative HPLC runs were performed using a prepacked HILIC column from Waters with an inner diameter of 30 mm and a length of 100 mm or a hydrophilic endcapped column (Atlantis D-C18) also from Waters with the same column size. The mobile phase was acetonitrile in water with 0.1% formic acid. The flow rate was 40 mL/min. The sample was dissolved in acetonitrile or an acetonitrile–water solution. The sample solution was then filtered before injecting into the system.
SFC systems: The analytical SFC systems used were Berger systems from Thar Technology (Pittsburg, Pennsylvania). One analytical SFC system contained six chiral columns including: ChiralPAK-AD, ChiralPAK-AS, Chiralcel-OD, Chiralcel-OJ, Whelk-O, and ChrialPAK-IA columns (Chiral technology, Exton, Pennsylvania). Six solvent systems were used on these columns. These solvents were methanol, ethanol, isopropanol, acetonitrile, methanol with 0.2% isopropylamine, and acetonitrile with 0.2% trifluoroacetic acid. Another analytical SFC system contained six achiral columns including: Pyridine, Benzamide, Cyano, Diol, Silica, and Pyridine amide (Princeton, Cranbury, New Jersey). Six solvent systems also were used on these achiral columns including: ethyl acetate, acetonitrile, methanol, ethanol, methanol with 0.2% isopropylamine, and acetonitrile with 0.2% trifluoroacetic acid. The flow rate was 2.5 mL/min. Pressure was 120 bar and the temperature was 40 °C. A gradient of 5% to 50% cosolvent was performed on these columns for screening purposes.
Preparative SFC runs were performed on an Investigator system (Thar Technology) using various preparative columns with a diameter of 10 mm and a length of 250 mm. The mobile phase varied in each experiment. The flow rate was 10 mL/min. Samples were dissolved in mobile phase and then filtered before being injected into the system.
Results and Discussions
Retaining polar compounds using HILIC: Our laboratory received several polar samples. From the initial analytical column screening results, the samples had little or no retention on the columns. Therefore, the separation method was redeveloped on the HILIC or type-C columns. The first example was a very polar compound with no retention on reversed-phase C18 columns. The crude sample weighed ~1.0 g and was soluble in acetonitrile. DMSO was not used on the HILIC column because it also could be retained on the column for ~ 2–3 column voids resulting in coelution. The sample can be dissolved in 100% acetonitrile, a water–acetonitrile mixture, or methanol. The compound had little absorption at 254 nm, but significant absorption at 210 nm. The initial purity of the desired peak was ~ 55% by HPLC area at a wavelength of 210 nm. The compound contained an amino group and a carboxylic acid group with no aromatic structure presented. An inversed gradient from 100% acetonitrile to 65% acetonitrile with 0.1% formic acid was performed on the HILIC column. It was discovered that the compound had good retention and also good resolution on the HILIC column. The result is displayed in Figure 1. From Figure 1, the desired peak (peak 1) is well separated from the peaks of other two impurities. All three peaks were retained on the HILIC column with a retention time of 3.5–6.0 min. A total of ~ 0.45 g of the purified material was recovered. The purity was better than 98% on the detection wavelength at 210 nm. The yield was ~ 82%. This yield is defined as the desired component yield ([the amount of the desired component recovered after the purification/the amount of the desired component present in the crude] x 100%).
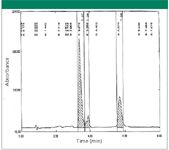
Figure 1: The retention of polar compound on a HILIC column in acidic condition.
The second example was also a very polar compound with little retention on the regular reversed-phase columns. The sample mass was ~ 100 mg. The sample contained an aromatic group and an ester group. The initial purity of the desired peak was ~ 80% by HPLC area at a wavelength of 210 nm. Synthetic chemist noted that this sample was unstable in the presence of acid or base modifiers. Therefore, a HILIC column in neutral condition was used for purifying the compound. It also was discovered that HILIC column could not be stable at either highly basic conditions or highly acidic conditions (pH < 2). This sample was dissolved in acetonitrile at a concentration of ~ 50 mg/mL. The column was loaded with a good amount of sample (~ 45 mg) for each injection. The impurities also were separated. Three injections were made. The results are shown in Figure 2. In Figure 2, the desired peak is the main peak. All the peaks were retained on the column. ~ 58 mg of the purified material was obtained at a purity of better than 97% by HPLC area at a wavelength of 210 nm. The yield was ~ 73%.
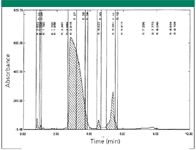
Figure 2: The separation of sample with a HILIC column under neutral condition.
Retaining polar compounds using hydrophilic endcapping columns: Some polar compounds also were retained on hydrophilic endcapped columns. Water (100%) can be used as the mobile phase on these columns. However, the columns should be washed with 100% acetonitrile to prevent the phase collapse before running the 100% water on the column. One example obtained was a polar compound with multiple amine groups and an aromatic group. The sample was already in DMSO solution and estimated a crude weight of ~ 200 mg. The initial purity was ~ 30% by HPLC area at a wavelength of 210 nm. Because the sample was under urgent request, a rapid turn around time was required. A HILIC column was not a good choice because DMSO also could be retained on the column. Therefore, a hydrophilic endcapped column (D-C18) was used for purification. The results are shown in Figure 3. In Figure 3, the desired peak is the third peak. The first peak is the DMSO peak. A gradient with a holding time of 5 min at 100% water was used to elute the desired peak. Then, a quick gradient to 100% acetonitrile was used to elute the rest of the impurities. The column was loaded with a significant amount of sample (20 mg) for each injection. A total of 10 injections were made. Approximately 46 mg of the purified material was obtained at a purity of better than 95% by HPLC area at a wavelength of 210 nm. The yield was ~ 75%.
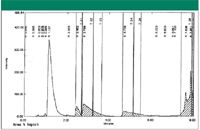
Figure 3: Purification with a hydrophilic endcapped column.
SFC for achiral purifications: SFC is one of the most successful methods for separating chiral molecules. Recently, it has gained acceptance for achiral purifications in drug discovery research in the pharmaceutical industry. With faster separation, higher sample throughput, less organic solvent usage, and normal-phase mode purification, SFC technology is sometimes more advantageous than reversed-phase HPLC–MS technology for achiral purifications. However, SFC still lacks mass-triggered detection and system reliability, which makes it difficult to use SFC as a primary purification tool for achiral samples. In our laboratory, the SFC systems were used mainly for chiral separations and as a back-up process for achiral separations of large samples, unstable samples, and samples with no resolution on HPLC–MS systems.
A few of the samples in our laboratory had little or no separation from the initial reversed-phase HPLC screening columns. Therefore, SFC was used for the separation. The first example was a diastereomer with two stereo-centers. Its stereo-center contained a pyridine group and a chlorinated group. The sample mass was ~ 500 mg. Various chiral stationary phases were screened. It was found that a total of four peaks (one pair of isomers for each diastereomer) could be isolated on the ChiralPAK-AD-H column. The results are shown in Figure 4. A gradient of 5–30% of methanol over 8 min at 120 bar and 40 °C was used for the separation. All four peaks were isolated. Approximately 10 mg of crude sample was loaded on a preparative ChiralPAK-AD-H column (250 mm x 10 mm) at each injection. A total of 50 injections were made. The isolated second peak had a purity of ~ 90%. The other three peaks had a purity of > 98%.
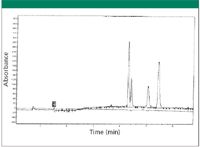
Figure 4: Two pairs of diastereomers separated on an SFC chiral column.
The second example was a regioisomer containing an aromatic group and a secondary amine group. The sample mass was ~ 2g. No separation on the reversed-phase HPLC column screening was obtained. However, separation did occur and was discovered after screening through SFC achiral columns. The best separation was evident on the 2-ethylpyridine column with 20% methanol as cosolvent at 120 bar and 35 °C. The results are illustrated in Figure 5. In Figure 5, the two regio-isomers are in 1:2 ratios. Both peaks were separated on an SFC achiral column. Approximately 30 mg of sample was loaded on the preparative column (250 mm x 10 mm) at each injection. A total of 70 injections were made. The purity of the isolated isomer was ~ 99% for first peak and ~ 98% for the second peak.
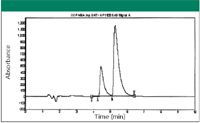
Figure 5: Regioisomer separation on an achiral column of 2-ethylpyridine with SFC.
Conclusions
The process of using several different approaches to address the difficult samples was successful. These approaches can be used to facilitate discovery purification workflow by reducing method development and purification time. Using normal-phase SFC technology for singleton achiral purification helped resolute structurally similar components and also reduced the chance of degradation in aqueous solution. Specialty columns such as HILIC or hydrophilic endcapped columns were able to retain polar components and make the separation work feasible.

Acknowledgments
The authors would like to thank Mike Carta, Kevin Barry, and Cortina Santo for their contributions and assistances.
Reference
(1) L. Miller, C.M. Grill, T. Q. Yan, and O. Dapremont, J. Chromatogr.1006, 267 (2003).
(2) E.R. Francotte, Switz. Chimia51, 717 (1997).
(3) C.M. Grill, L Miller, and T.Q. Yan, J. Chromatogr.1026, 101 (2004).
(4) J.H. Kennedy, M.D. Bevo, and J.D.Williams, J. Chromatogr.1026, 101 (2004).
(5) T.A. Berger, J. Smith, K. Fogelman, and K. Kruluts, Am. Lab.34, 14 (2002).
(6) J.J. Pesek and M.T. Matyska, LCGC26(1), 20 (2008).
(7) G. Terflorth, J. Chromatogr.906, 301 (2001).
(8) C.J. Welch, W.R. Leonard Jr., J.O. Dasilva, M. Biba, J. Albaneze-Walker, D.W. Headerson, B. Liang, and D.J. Mathre, LCGC23(1), 15 (2005).

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.












