Anomalous Retention Prediction Using Modelling Software in Gradient Reversed-Phase Liquid Chromatography: Why it Can Occur and How to Prevent It
This article presents the benefits of screening column and mobile phase combinations that generate differing chromatographic selectivities in reversed-phase gradient ultrahigh-pressure liquid chromatographic (UHPLC) method development strategies. Photodiode array (PDA) and mass spectrophotometric (MS) detection was necessary to facilitate peak tracking or identification of components in the sample mixture to build retention models. Retention time prediction accuracies of < 0.3% were obtained from a two-dimensional gradient time vs. temperature model when the initial gradient conditions were maintained. However, anomalous retention predictions were observed when higher %B initial gradient conditions were employed. Polar analytes in the sample mixture that started to migrate down the column in the dwell volume of the UHPLC system produced inaccurate retention time predictions if an inappropriate dwell volume was used in the retention model. An iterative dwell volume estimation was demonstrated to generate more accurate retention time predictions than when a practically determined dwell volume was used. However, to obtain good predictions the analyst should endeavour to use initial chromatographic conditions that promote focusing of all analytes on top of the column (that is, retention factor > 10).
A popular strategy in reversed-phase liquid chromatographic (RPLC) method development for small-molecular-weight active pharmaceutical ingredients (APIs) is to screen various stationary and mobile phase combinations using a fixed %B/min gradient and temperature (classed as Wave 1, see Figure 1) using automated column and mobile phase screening technologies (1). The most promising stationary and mobile phase combination based on the number of peaks detected, peak shape, and resolution is then taken through to Wave 2 (see Figure 2), in which a two-dimensional gradient time vs. temperature resolution model is constructed. In the case of ionizable compounds, it may be beneficial to screen mobile phases of low, intermediate, and high pH in Wave 1 to completely deprotonate or protonate the analyte(s). To obtain a robust method, mobile phases with aqueous pH values > ±2 pH units away from the pKa of the analyte(s) should ideally be used. In so doing, differing retentivity and selectivity may be acquired. In most instances, the authors do not recommend modelling pH in Wave 2 where differing selectivity would only occur over a narrow pH range close to the pKa of the analyte(s), which typically would result in poor method robustness. In addition, the modelling of pH, even over a narrow pH range, requires a relatively large number of input experiments.
From the resultant resolution model, the optimum separation conditions can be predicted. A recent project was undertaken to provide educational material for the promotion of such a method development strategy using pre-selected analytes that would highlight the following prerequisites: 1) peak tracking using a combination of photodiode array (PDA) and mass spectrophotometric (MS) detection; 2) the ability to switch the elution order of analytes based on using differing organic modifier/ stationary phase combinations; and 3) the ability to optimize the separation in terms of fine-tuning the percentage organic of the initial, final mobile phase compositions and gradient time.
During the optimization step there is always a tendency for the chromatographer to speed up the analysis by increasing the initial %B (that is, the mobile phase containing the high organic content). While this is a valid approach, particularly if the %B/min is kept constant to maintain the same desired chromatographic selectivity, if too high a %B is chosen, then the polar analytes in a RPLC separation may start to migrate in the dwell volume and may not be focused on top of the column. If this is the case, this can result in discrepancies between the predicted and experimental retention times unless a more accurate dwell volume value is used in the retention model.
Experimental
Water, methanol (MeOH), and acetonitrile (MeCN) used were of LC– MS grade and were supplied by Romil. All compounds used in this study and the mobile phase additives (ammonium formate and formic acid) were supplied by Sigma Aldrich. Ultrahigh-pressure liquid chromatographic (UHPLC)-PDA analysis was performed on a Shimadzu Nexera XS UHPLC (Shimadzu UK Ltd) equipped with two binary pumps (LC-40D XS) and proportionating valves, degassers (DGU-405), flow-through needle autosampler (SIL-40C XS), column oven (CTO-40C), diode array detector (SPD-M30A) with a 1 μL/10 mm pathlength flow cell, 40 μL mixer (practically determined dwell volume = 353 μL and system volume = 14 μL [2]), single quadrupole mass spectrometer (LCMS 2020) with a Shimadzu electrospray ion source working in the positive ionization mode, communication bus module (CBM-40lite), and a six-position column switching valve. Selected ion monitoring of the M+H+ peaks was used to track the components. The system was controlled and data collected using LabSolutions software (Shimadzu UK Ltd, version 5.114). Retention modelling and log D value determinations were performed with ACD/LC Simulator and ACD/Percepta software (versions 2021.2.2), respectively. At least 20 column volumes of the appropriate mobile phase were flushed through the columns prior to commencing the testing, or on changing the mobile phase conditions.
Wave 1 Chromatographic Conditions (see Figure 1):
Totally porous Avantor ACE Excel C18, ACE Excel C18-AR, and ACE Excel CN-ES columns (all 100 × 3 mm, 3-µm, 100 Å) were supplied by VWR Avantor. Stock 100 mM aqueous ammonium formate was prepared by dissolving 6.306 g of ammonium formate in 900 mL of water, adjusting the pH to 3.0 using formic acid, and then made up to 1000 mL with water. The degassed mobile phases A and B corresponded to 10 mM aqueous ammonium formate (pH 3.0) in water and 10 mM aqueous ammonium formate in 9:1 (v/v) MeCN–water or 9:1 (v/v) MeOH–water, respectively. Unless otherwise stated, the following UHPLC conditions were used flow rate 0.43 mL/min, temperature 40 °C, 1 µL injection volume, linear gradient 5–95% B over 10 min, hold at 95% B for 1 min, linear gradient 95–5% B over 0.1 min, and hold at 5% B for 9.9 min to equilibrate the column. The first baseline disturbance for a water injection was used as the dead time (tM) marker. The PDA detector was set to monitor a wavelength of 254 nm (bandwidth 8 nm), with a reference at 360 nm (bandwidth 100 nm). The data sampling rate was set at 40 Hz. The mass spectrometer utilized positive polarity mode electrospray ionization, and used 1.5 L/min nebulizing gas flow and 15 L/min drying gas flow. The interface temperature was set to 350 °C, the desolvation line (DL) temperature 250 °C, and heat block temperature 200 °C.
Wave 2 Chromatographic Conditions (see Figure 2):
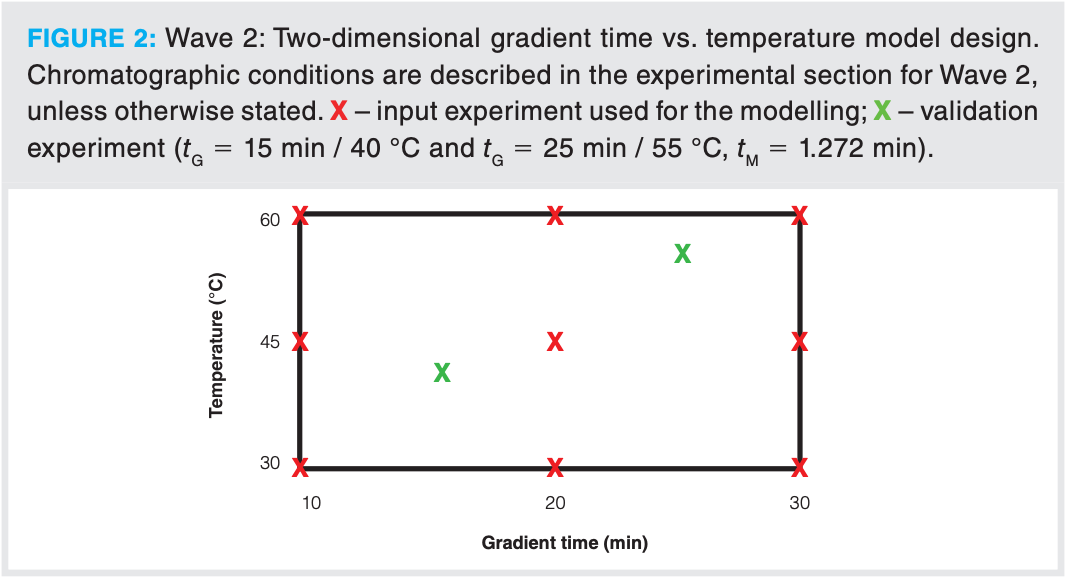
Conditions as described in Wave 1 were used unless otherwise specified. The Avantor ACE Excel C18-AR column and MeCN were used to construct a two-dimensional 3 × 3 (that is, nine input experiments) gradient time vs. temperature (tG vs. T) retention model (3). Temperatures of 30, 45, and 60 °C were examined at gradient times of 10, 20, and 30 min. The integrity of the input data was established by repeating the initial conditions at the beginning and end of the batch.
Results and Discussion
Method Development Activity: Wave 1:
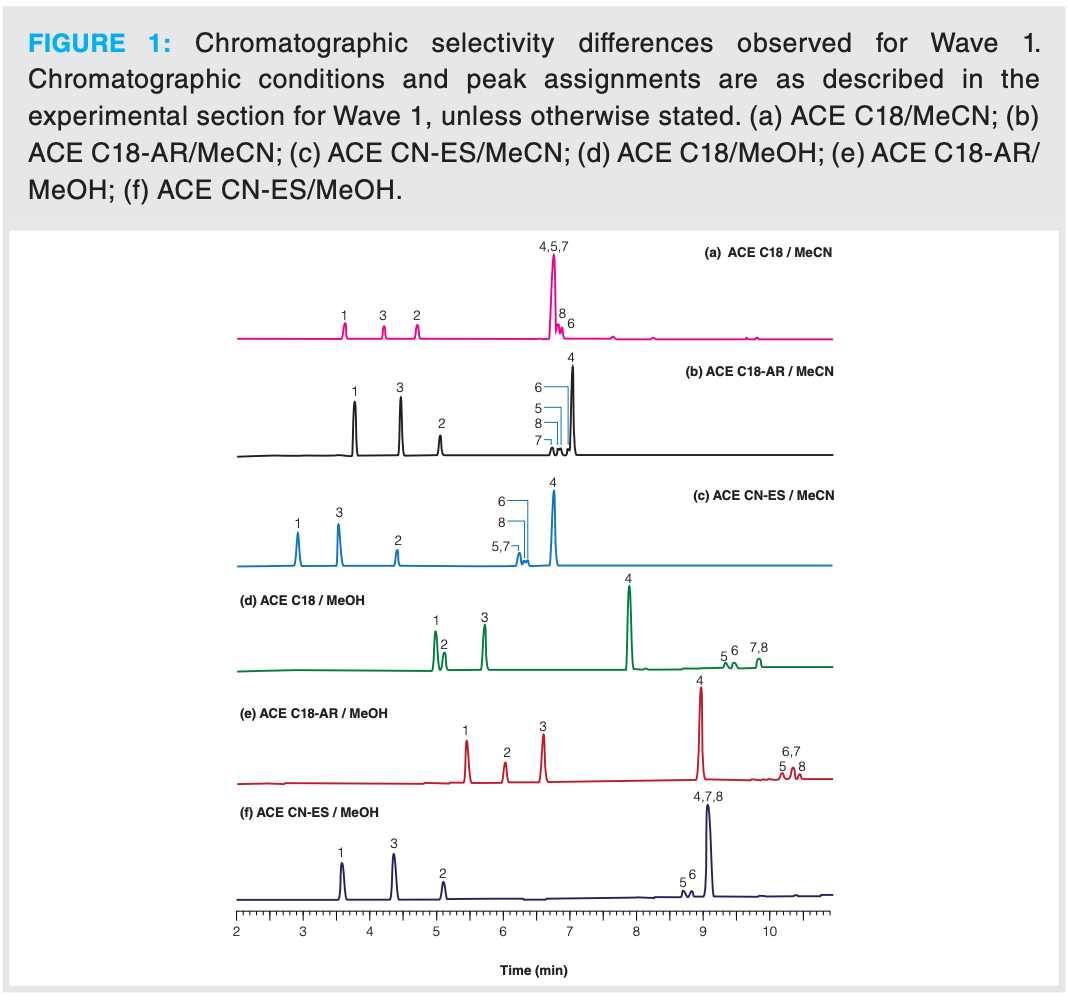
The aim of this work was to demonstrate the large selectivity differences that can be exploited when differing stationary phases (C18, C18 with aromatic selectivity, and a cyano with high hydrophobic character) are combined with differing organic modifiers (MeOH and MeCN) in gradient RPLC at a fixed gradient slope of 9 %B/min and a column temperature of 40 °C using column dimensions of 100 × 3 mm, 3-µm. This dimension is popular because it is not significantly affected by the system volume of the liquid chromatograph and can still provide a relatively low solvent consumption. A 10 mm ammonium formate buffer (pH 3) was maintained in both mobile phases A and B and a flow rate of 0.43 mL/min was employed because this was ideal for mass spectrometry with an electrospray ionization source.
The test mixture was composed of eight analytes (1, theophylline; 2, sulfamerazine; 3, caffeine; 4, sulfaquinoxaline; 5, prednisone; 6, cortisone; 7, prednisolone; and 8, hydrocortisone), all of which possessed varying hydrophobicity (that is, log D at pH 3 ranged between 0.09–1.66). Sulfaquinoxaline was selected to represent the API, as ideally this should elute after the other components. MS could not discriminate between cortisone and prednisolone, as they both possess the same m/z charge ratio; however, their PDA spectra differed significantly enough to allow discrimination of these components. In contrast, the steroidal pairs prednisolone/ hydrocortisone and prednisone/ cortisone had similar PDA spectra but could be discriminated via their different m/z charge ratios. Therefore, by using a combination of PDA and MS all the components of the mixture could be peak tracked successfully.
From Wave 1, it could be concluded that the column possessing a C18 with aromatic functionality and MeCN was the most promising combination, as all eight components could be observed with good peak shape, with sulfaquinoxaline eluting last (see Figure 1). Large selectivity differences in the elution profile between MeOH and MeCN could be observed across both C18 columns; sulfamerazine eluted before caffeine with MeOH, while the reverse was true for MeCN. This observation was not seen with the cyano column with enhanced hydrophobicity. In addition, with MeOH sulfaquinoxaline (4) eluted before the four steroidal components, whereas with MeCN it eluted much later.
Method Development Activity: Wave 2:
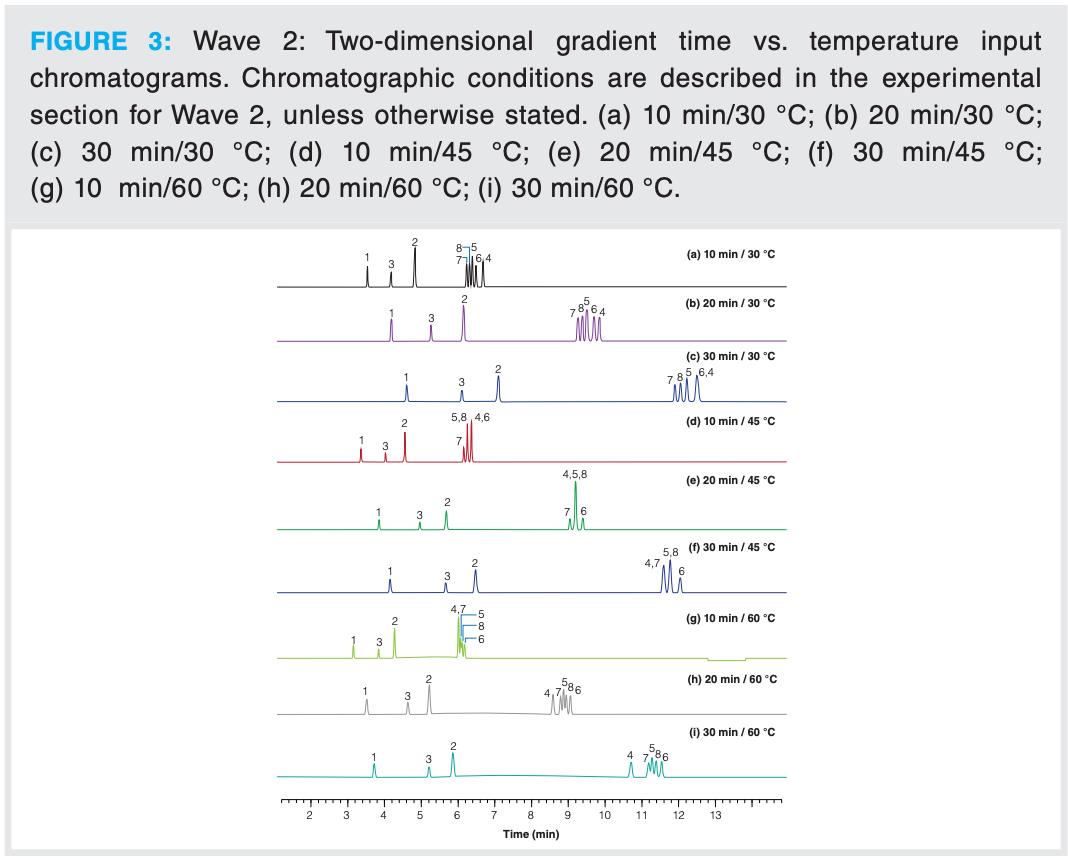
A 3 × 3 (nine input experiments) gradient time vs. temperature (tG vs. T) retention model (3) was constructed using the C18 column with aromatic functionality and MeCN as the organic modifier (see Figure 2). The same initial and final starting %B and flow rate was employed as in Wave 1. As can be observed in Figure 3, there is considerable peak movement as a function of temperature and gradient time. Peak tracking was performed using positive ion MS using an electrospray ionization source and PDA spectroscopy.
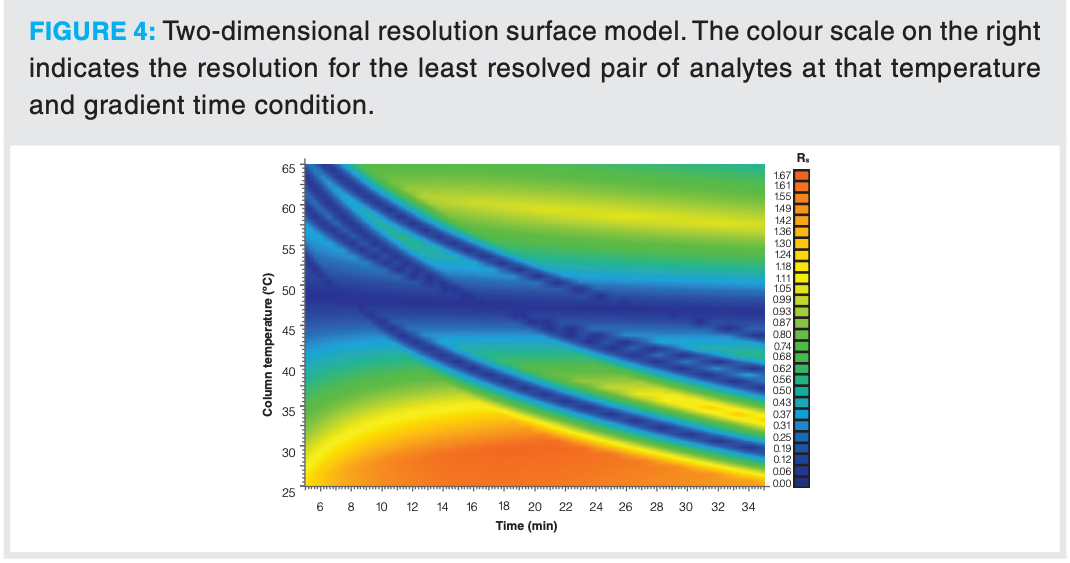
The input data were transferred to the retention modelling software and a resolution response surface was constructed (see Figure 4, VD = 353 µL, tM = 1.27 min). The percentage difference between the actual and predicted retention time (%ΔtR) was found to be in excellent agreement (%ΔtR < 0.3%) for the validation conditions, confirming the accuracy of the retention model. Improved separation was achieved at temperatures below 35 °C. The presence of dark blue regions corresponding to poor resolution sandwiched between orange and green colours (higher resolution) indicated that there are switches of peak elution order.
The Problem: Discrepancies Between Actual Retention Times and Predictions:
From the two-dimensional model of the dependence of retention on gradient time and temperature (see Figure 4), the elution conditions (that is, temperature, initial and final %B in the gradient, and the gradient slope) can be adjusted to produce an optimized separation. By investigating the retention behaviour of the analytes in the test mixture using the retention modelling software, it could be predicted that a tG of 10 min with an initial to final %B of 35 to 50 %B would yield an acceptable separation (see Figure 5[a]). However, surprisingly, the retention prediction did not match with the actual retention times (that is, %ΔtR values between 5–18% were obtained, see Figures 5[a] and [b]), even though validation runs were in excellent agreement (%ΔtR values < 0.3%). Similar observations were also encountered using different UHPLC instrumentation, differing types of stationary phase, and with a different type of test mixture.
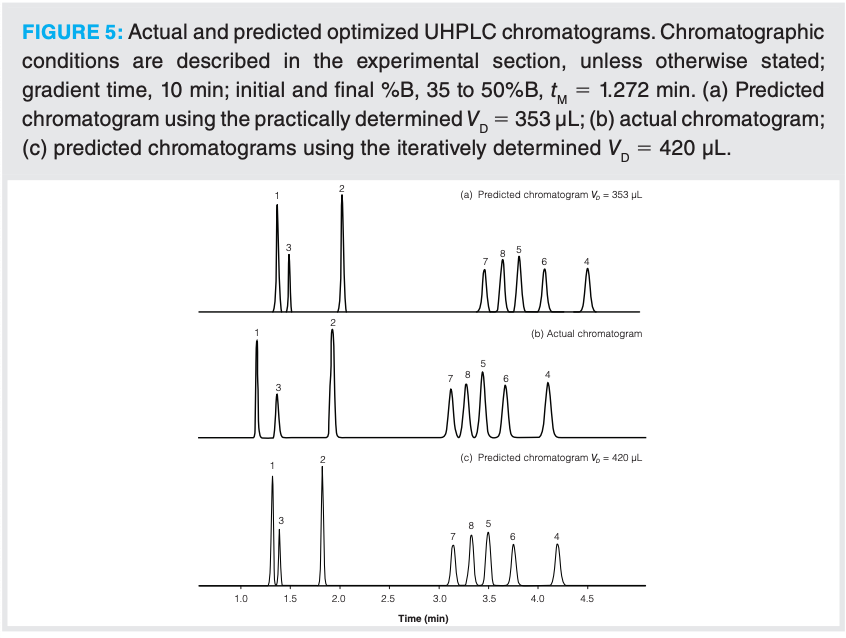
The dwell volume of a UHPLC instrument is an input variable that the retention model uses to predict analyte retention. It is defined as the volume from the point at which the mobile phases first mix in the pump to the head of the column. It can be determined in different ways; in many earlier publications it is suggested that a wide linear gradient range should be employed accompanied with a high flow rate (4,5). In comparison, several instrument manufacturers utilize a step gradient with a low flow rate and a narrow organic range. It has previously been observed that the gradient type (step or linear), flow rate, and gradient range are all critical for the accurate estimation of dwell volumes (differences of up to 80% have been observed [2]). It is the authors’ opinion that the dwell volume should be determined using gradient conditions that are appropriate for the type of analyte and LC instrumentation that will be used (reference 2 describes a procedure for the estimation of dwell volumes suitable for modelling purposes). Since the dwell volume determination is based on a linear gradient, it can also be used to ensure that linear gradients can be generated by the UHPLC instrumentation. The dwell time volume was estimated at a flow rate of 0.43 mL/min to be 353 µL, as described in reference 2, and was used in the retention model. It has been previously demonstrated (2,6,7) that even relatively large errors (up to ±20%) in the estimation of the dwell volume used in the retention model do not have a significant impact on the accuracy of the predicted retention times (that is, < 1%).
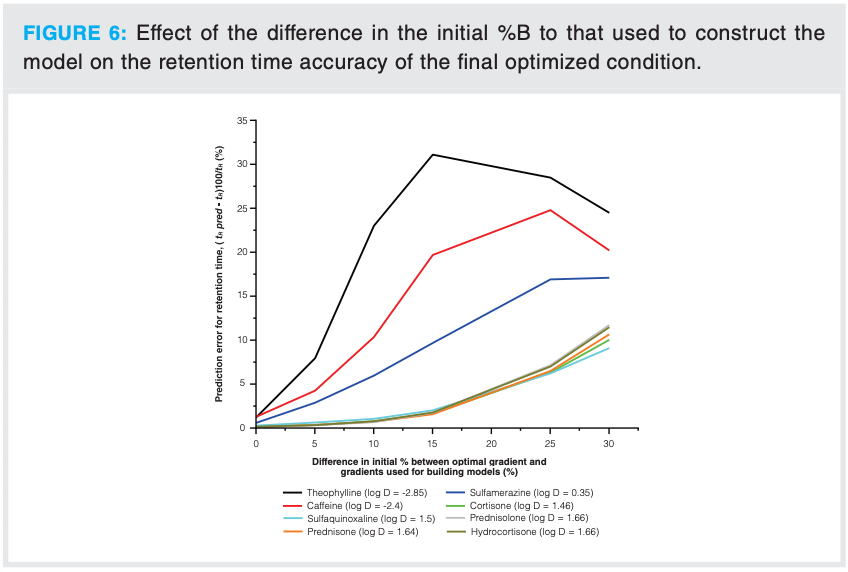
Further investigations highlighted that excellent retention time predictions could be achieved for this and other test mixtures when the initial %B was kept the same as that used to generate the retention model input data; however, when higher %B conditions were used, inaccuracies in retention time prediction became significant. The degree of error in the retention time predictions was observed to be related to the difference in initial %B of the optimized method relative to that used to construct the model (that is, when Δ%B initial [%B initial optimized – %B initial used in the model] was large, poorer retention predictions were observed, see Figure 6). The more polar analytes exhibited the phenomenon to a greater extent compared to the more nonpolar analytes. This was also shown to be the case with an additional test mixture of phenone standards.
The Solution:
An alternative approach for estimating the dwell volume—as well as to compensate for errors in other parameters—is to iteratively try a few different dwell volumes while fitting the retention model, compare the residuals obtained, and, based on this, select the dwell volume that gives the lowest residual (8). This has been implemented recently in the software that we have used (9). When the retention times for the test analytes for the 30 °C input data were used in this iterative approach, the dwell volume was estimated to be 420 µL instead of the experimentally estimated values of 353 µL. When this dwell volume was used in the retention model, a much better retention prediction accuracy was achieved (< 2.5% for the steroids, however, the more polar analytes still gave larger errors), see Figures 5(b) and 5(c).
While it is true that relatively large inaccuracies in the dwell volume do not impact on the retention time prediction accuracy when initial %B remains the same as (or lower than) that used to construct the retention model, this is not the case when higher initial %B conditions are employed (that is, the analytes are not focused on top of the column and start to migrate isocratically down the column).
If the analyte is focused on top of the column (that is, it possesses a large isocratic retention factor [k]) at the higher %B initial conditions, this phenomenon is not observed, and dwell volume accuracy is not that critical. However, if analytes are of a low to medium retentivity (low hydrophobicity when considering a reversed-phase mechanism, low isocratic k values) and therefore are not focused on top of the column and start to migrate down into the dwell volume, then this phenomenon is profound, particularly if an inaccurate dwell volume is used.
It appears that the established methods for determining dwell volumes are not appropriate for accurate retention time predictions and that the iterative methodology generates a more meaningful estimation for retention modelling purposes.
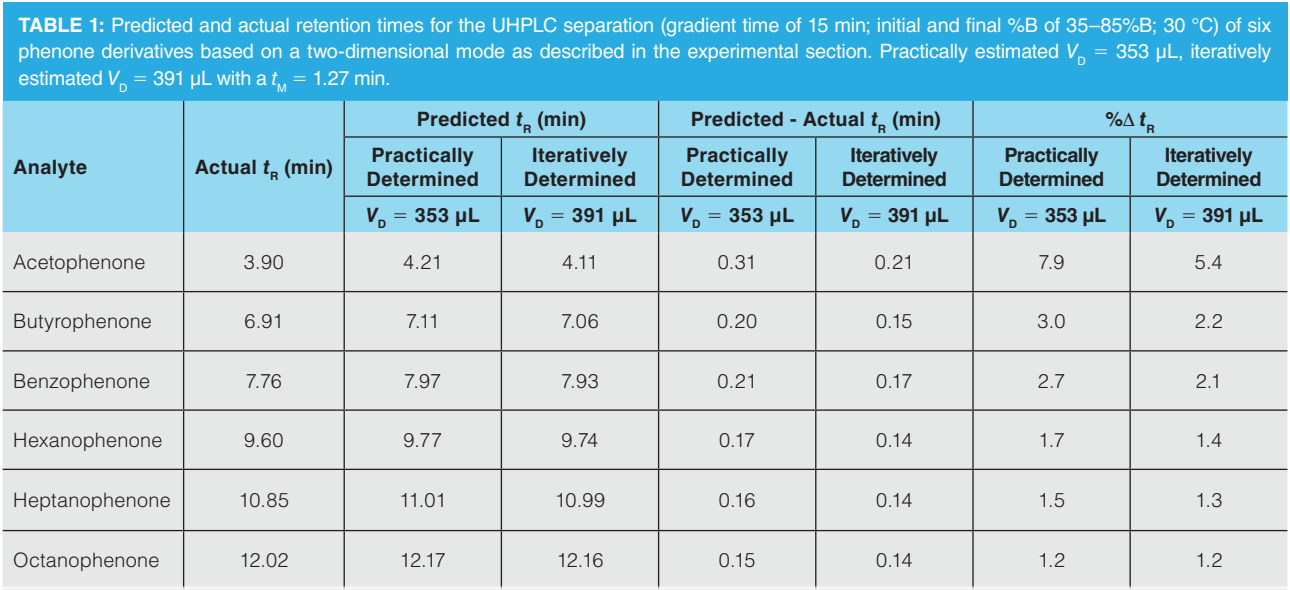
To investigate this further, a test mixture of six phenones of varying hydrophobicity was evaluated. Table 1 demonstrates that the inaccuracy of the retention prediction of the six phenones becomes less pronounced as a function of the analytes’ retentivity on the column when the practically determined dwell volume (353 µL) was used to construct the model. However, when the iteratively estimated dwell volume was used (391 µL), acceptable retention time predictions were obtained for all the phenones (%ΔtR values < 2.2%), except for the more polar acetophenone, which was least retained.
From the log k vs. %B relationship it is possible to predict the retention factor of the analytes at differing %B conditions. For the eight-component test mixture all components had k values <3 when run isocratically at 35%B, and generated poor retention predictions if an inappropriate dwell volume was used. For this relatively polar mixture of components, Figure 6 suggests that using an initial Δ%B > 10%B (that is, 15%B initial concentration) should not be considered if acceptable retention predictions are required. In comparison, for the phenone mixture the more hydrophobic components such as benzophenone, hexanophenone, heptanophenone, and octanophenone possessed k values > 10 at 35%B and generated acceptable retention prediction accuracy of ≤ 2%.
Conclusions
This article has highlighted the usefulness of method development strategies that 1) utilize the complementary chromatographic selectivity of differing stationary and mobile phase combinations to screen initial conditions (Wave 1), 2) the necessity of combining MS and PDA detection for peak tracking purposes, 3) the power of two-dimensional gradient time vs. temperature retention modelling (Wave 2), 4) the high retention time prediction accuracy of gradient RPLC within the resolution model, and 5) the ability to optimize the separation further by refining the % initial, final, and gradient time. It has also been surprisingly demonstrated that small errors in the dwell volume used for the retention model may give rise to large retention prediction inaccuracies depending on the retentivity of the analyte. It is therefore recommended that an iterative methodology for estimating dwell volume should be used, as this is a more accurate reflection of the true dwell volume than the well-established approaches previously recommended (2,4,5).
If the analyte is retained on top of the column (a process often referred to as peak focusing or compression), then this anomalous retention prediction inaccuracy is not observed, and the accuracy of the dwell volume estimation is not critical. It is recommended that the isocratic retention factor must be >10 at the predicted initial %B composition. It is anticipated that a warning message in newer versions of retention modelling programmes will be incorporated that will highlight if a too high initial %B condition is selected, which may result in unacceptable retention prediction errors.
Analytes of high to medium polarity may not be focused on top of reversed-phase columns and will start to migrate down the column in the dwell volume; therefore, if an incorrect dwell volume is used, the software will not model the retention properly, which may result in large retention prediction errors along with possible selectivity differences, as can be seen in Figure 5. As an approximation for accurate retention time predictions (ΔtR < 2%), it is recommended that the optimized initial gradient conditions should correspond to the %B initial used to construct the model, plus no more than 10% MeCN (or that analytes should ideally possess isocratic k values >10).
Acknowledgements
Special thanks to Shimadzu for providing the UHPLC instrumentation used within this study. We thank VWR Avantor for supplying the columns required for this work. Finally, we thank Tom Blackadar, Graham McGibbon, and Anne Marie Smith of ACD/Labs for their participation in the project and for provision of software.
References
(1) Euerby, M. R.; Fever, M.; Hulse, J.; et al. Maximization of Selectivity in Reversed-Phase Liquid Chromatographic Method Development Strategies. LCGC Eur. 2016, 29, 8–21.
(2) Petersson, P.; Boateng, B. O.; Field, J. K.; Euerby, M. R. A Practical Approach to Modelling of Reversed-Phase Liquid Chromatographic Separations: Advantages, Principles, and Possible Pitfalls. LCGC Eur. 2018, 31, 120–143.
(3) Petersson, P.; Munch, J.; Euerby, M. R.; et al. Adaption of Retention Models to Allow Optimisation of Peptides and Protein Separations. Chromatography Today 2014, 15–18.
(4) EDQM, EurPh, Section 2.2.4.6 Chromatographic Separation Techniques, 7.0th Ed. (EDQM, Strasbourg, France).
(5) Snyder, L. R.; Dolan, J. W. High Performance Gradient Elution: The Practical Application of the Liner-Solvent Strength Model; John Wiley & Sons, 2007.
(6) Snyder, L. R.; Quarry, M. A. Computer Simulation in HPLC Method Development. Reducing the Error of Predicted Retention Times. J. Liq. Chromatogr. Relat. 1987, 10, 1789–1820. DOI: 10.1080/01483918708066799
(7) Ghrist, B. F. D.; Cooperman, B. S.; Snyder, L. R. Design of Optimized High-Performance Liquid Chromatographic Gradients for the Separation of Either Small or Large Molecules: I. Minimizing Errors in Computer Simulations. J. Chromatogr. A 1988, 459, 1–23. DOI: 10.1016/S0021-9673(01)82014-8
(8) Lundell, N. Implementation and Use of Gradient Predictions for Optimization of Reversed-Phase Liquid Chromatography of Peptides. Practical Considerations. J. Chromatogr. A 1993, 639, 97–115. DOI: 10.1016/0021-9673(93)80245-4
(9) ACD/Labs Software. www.acdlabs.com (accessed 2023-01-17).
About the Authors
Jennifer K. Field is a research scientist at Shimadzu Centre of Excellence.
Melvin R. Euerby is principal scientist at Shimadzu Centre of Excellence and visiting professor at the Open University and the University of Strathclyde.
Patrik Petersson is a principal scientist at Ferring Pharmaceuticals.
Stuart N. Berry is an application scientist at Advanced Chemistry Development, UK Ltd. (ACD/Labs).
James Hogbin is an application scientist at Advanced Chemistry Development, UK Ltd. (ACD/Labs).
Veronica Paget is an account manager, UK, and Ireland at Advanced Chemistry Development (ACD/Labs).
Earl McKoy is a senior product specialist in LC at Shimadzu UK.
Raymond Wong is national sales manager at Shimadzu UK.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.












