Analysis of siRNA with Denaturing and Non-Denaturing Ion-Pair Reversed-Phase Liquid Chromatography Methods
In this paper, we describe denaturing and non-denaturing ion-pair reversed-phase liquid chromatography (RPLC) methods for analyzing small interfering ribonucleic acid (siRNA). Drug formulations consisting of one or two siRNA duplexes were analyzed in non-denaturing conditions at a low column temperature to separate intact duplexes from single-stranded oligonucleotide contaminants and quantify them. In a denaturing method, we used an elevated column temperature to ensure the denaturation of siRNA duplexes into their single-stranded oligonucleotide counterparts. The goal of the denaturing LC method was to investigate the impurities in daughter oligonucleotides in siRNA. A column with chemically modified C18 column hardware showed improvements in analytical performance for nucleic acids compared to a conventional C18 stainless-steel column with the same pore size.
Oligonucleotides and small interfering ribonucleic acid (siRNA) belong to a growing class of nucleic acid-based therapeutic compounds (1,2). siRNA molecules are short (~20–25 base pairs), chemically modified RNA duplexes capable of in vivo regulation of protein expression. The promise of these new modalities is that they have the potential to treat a broad range of diseases. There have been great advances in oligonucleotide manufacturing and drug delivery in the last decade. Until 2016, three oligonucleotides were approved by pharmaceutical authorities; however, the number of oligonucleotide therapeutics studies has since increased considerably (1,2). The proliferation of nucleic acid therapies in clinical trials created a demand for analytical methods suitable for quantitation and characterization of these compounds. The methods should be robust, reliable, and easy to perform in different environments and, in many cases, by non-expert analysts. This requirement conflicts with the considerable complexity of siRNA analysis. Quantitative measurement and characterization of the complex mixture is paramount for the safety and efficacy of the drug formulations.
Ion-pairing reversed-phase liquid chromatography (IP-RPLC) has been shown to be an efficient method for sensitive analysis of nucleic acids in both LC–UV and LC–mass spectrometry (LC–MS) setups (3–9). In this work, we describe drug formulation analysis comprising a single or two siRNA duplexes. siRNA can be analyzed either under denaturing conditions, when the duplex is transformed into two complementary oligonucleotides (10), or under non-denaturing conditions, as intact duplexes (11). The latter approach is used when it is desirable to estimate the excess of single-stranded oligonucleotides in the siRNA duplex formulation. Non-denaturing IP-RPLC assays play a pivotal role in the optimization of the annealing procedure following oligonucleotide synthesis (10). The denaturing IP-RPLC method uses a column temperature exceeding the melting temperature of the siRNA duplexes (10,12). The goal of this analysis is to resolve the complementary oligonucleotides from each other and from their synthetic impurities to verify the purity of the siRNA oligonucleotide constituents while identifying the impurities present in the drug formulation. The separation of synthetic impurities in single-stranded oligonucleotides is typically an easier task than the separation of complex impurities after their hybridization in a duplex siRNA form. The advantage of IP-RPLC over separation methods such as anion-exchange LC or polyacrylamide gel electrophoresis (PAGE) is that it is compatible with MS analysis (3,6,7,9). IP-RPLC MS can be used to confirm the molecular weight of oligonucleotide impurities or to confirm their sequence via tandem MS (MS/MS) sequencing (13,14).
Materials and Methods
Sample Preparation
A duplex solution of 0.40 mg/mL was prepared by dissolving the duplex siRNA C in Milli-Q water. This concentration is labeled as 100% standard solution “Duplex C.” For the construction of a calibration curve, a 200% stock solution was prepared (0.80 mg/mL); the investigated concentrations (130%, 100%, 70%, 8%, 4%, 2%, and 0.2%) were prepared by diluting this standard. Similarly, standards for non-denaturing method experiments were prepared using the mixture of siRNA Duplexes 1 and 2 at a concentration of 2.0 mg/mL in Milli-Q water, which represents the 100% standard; the final investigated concentrations were 130%, 100%, 70%, 6%, 4%, 2%, and 0.2%.
Instrument and Columns
An Acquity ultrahigh-pressure LC (UHPLC) H-Class Plus Bio system consisting of a quaternary solvent manager (QSM), column heater module (CM-A), active column preheater (APH), sample manager flow-through needle (SM-FTN) and photodiode array (PDA) detector equipped with 5-μL titanium cell, was used for the denaturing method. The same instrumentation setup—except with a PDA detector equipped with an analytical flow cell—was used for the non-denaturing method evaluations. Sample integration and quantitation was performed with Empower 3.0 software. The columns used for analysis were packed with the same chromatographic sorbent. The Acquity UHPLC Peptide BEH C18 column (300 Å, 1.7 μm, 2.1 mm × 150 mm) uses conventional stainless-steel column hardware (we will refer to this column as column #1), whereas the Acquity Premier Oligonucleotide BEH C18 column (300 Å, 1.7 μm, 2.1 mm × 150 mm) uses hardware modified with high-performance surfaces (HPS) for mitigation of the acidic sample adsorption on metal surfaces (15–18); we will refer to this column as column #2.
Denaturing and Non-Denaturing IP-RPLC Methods Conditions
For the denaturing process using IP-RPLC, a column temperature of 75 °C was used. Mobile phase A consisted of 0.07% (v/v) triethylamine (TEA) and 0.60% v/v 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) in Milli-Q water (which corresponds to 5 mM TEA and 60 mM HFIP aqueous solution). Mobile phase B was prepared by mixing 85% of mobile phase A with 15% of organic solvent consisting of methanol:acetonitrile mixture (70:30 v/v). The flow rate was 0.30 mL/min; the gradient went from 0 to 100% B in 20 min. More details are included in the literature (19). The peaks were detected by UV at 260 nm.
The non-denaturing IP-RPLC was performed at 20 °C. Mobile phase A consisted of 0.2% hexylamine (HA) and 0.5% of HFIP in Milli-Q water (this corresponds to 15 mM HA and 47.5 mM HFIP). Mobile phase B was a methanol:acetonitrile (80:20, v/v) mixture. The flow rate was 0.15 mL/min; the gradient went from 50 to 80% B in 30 min. More details are included in the literature (20). Both denaturing and non-denaturing IP-RPLC methods use mobile phases compatible with MS detection, which is useful for method development and peak identification of complex siRNA samples. UV detection at 260 nm was used for routine LC–UV siRNA analysis.
Results and Discussion
Denaturing LC Method for siRNA Oligonucleotides Analysis
In the first step, we screened multiple separation modes, columns, column lengths, and sorbent pore sizes (130 or 300 Å). Neither anion-exchange columns, nor C18 columns from several manufacturers used in IP-RPLC mode matched the resolution of siRNA sample achieved with column #1 (see the “Materials and Methods” section). This column was selected for further method development. However, when we started a systematic study with a new column #1, we observed significantly lower signal for siRNA oligonucleotides compared to the original column.
The sample recovery gradually improved for subsequent sample injections. This phenomenon has been described in the literature (15,17,18,21,22) for acidic molecules, such as adenosine triphosphate or oligonucleotides. It appears that siRNA oligonucleotides are adsorbed via ionic interactions on positively charged metal-oxide surfaces in a LC system flow path, and in particular, on column frits (17). Although the siRNA sample recovery improved for later sample injections, the non-specific adsorption is a concern for method robustness.
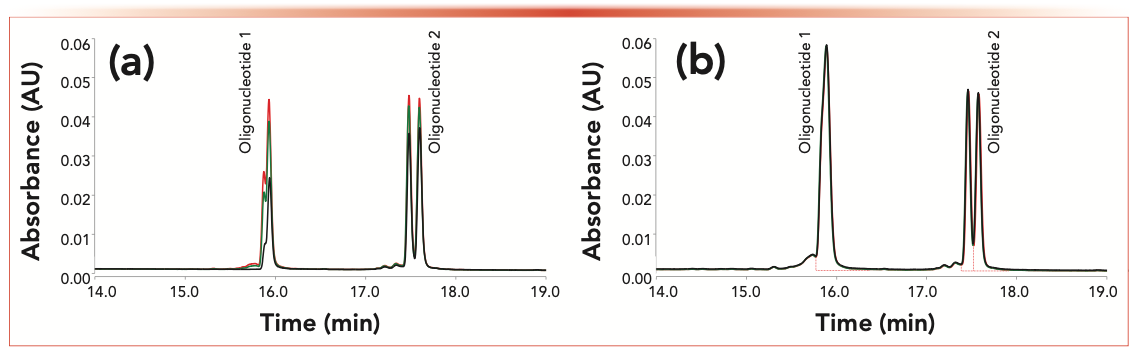
Therefore, we evaluated whether a novel column (column #2) can provide a better platform for siRNA analysis. According to the manufacturer, the column #2 hardware and frits are chemically modified with HPS surfaces to mitigate the undesirable sample adsorption (15,17,18,23). The results in Figure 1 illustrate the side-by-side comparison of the stainless steel column #1 and chemically modified hardware column #2. Both columns were packed with an identical sorbent. As the siRNA sample is analyzed under denaturing conditions, two main oligonucleotide groups are observed, resolved from each other and from their synthetic impurities that are eluted prior to each oligonucleotide. The dominant oligonucleotide 1 and oligonucleotide 2 peaks are resolved into their corresponding diastereoisomers (Figure 1); the diastereomers are created by phosphorothioate modifications of selected internucleotide linkages. Figure 1a illustrates the sample loss observed with a new stainless-steel column. The peaks in first injection (black chromatogram) were low; their height improved for second (green chromatogram), and third injection (red chromatogram). The results suggest that metal column hardware can be gradually conditioned with an excess of sample injected on column as reported in recent publications (16,17). Column #2 (Figure 1b) shows the same sample recovery for all injections 1–3; the overlapping chromatograms in Figure 1b are practically indistinguishable.
FIGURE 1: Three consecutive injections of siRNA duplex C on (a) a conventional stainless-steel column #1 and (b) on column #2. New out of the box columns were used for the siRNA analysis in denaturing LC method. In denaturing IP-RPLC conditions the siRNA duplex is converted into the oligonucleotides 1 and 2.
Improved siRNA Recovery with Column #2
Because of the promising results with column #2, we performed additional experiments summarized in Tables I and II. New out-of-the-box columns were used in all separate experiments. The peak area was calculated from UV 260-nm chromatograms by summing all dominant peak areas (groups at ~15.9 min plus 17.5 min) as indicated in Figure 1b, and the summed area was reported in the Tables I and II. Table I shows a gradual increase in peak area for the stainless-steel column for each subsequent sample injection. In contrast, the peak area for column #2 remained constant throughout the experiment and overall greater total area was observed.
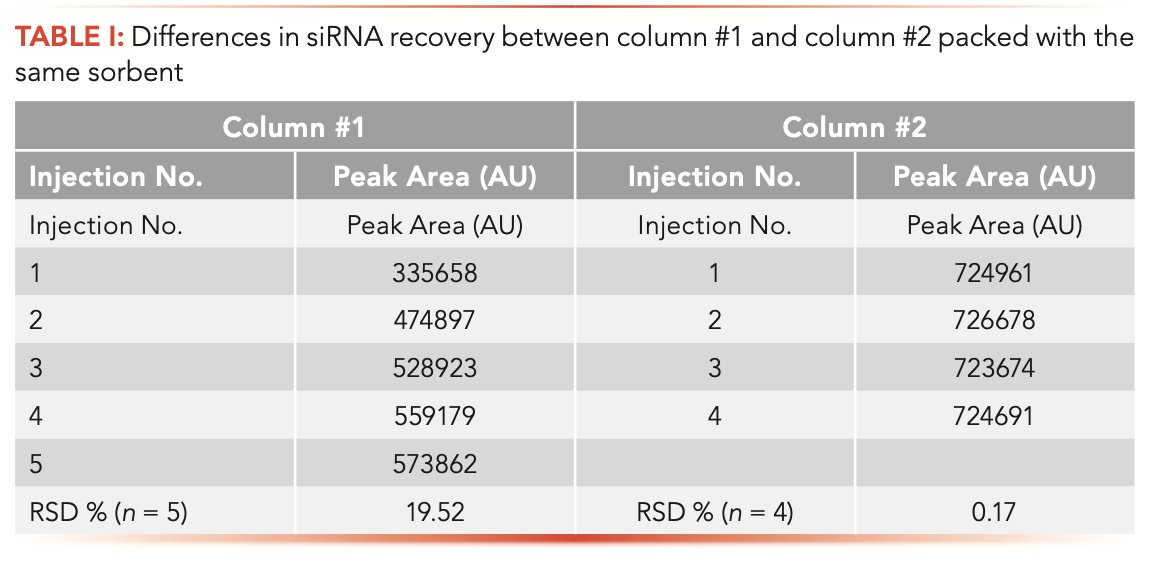
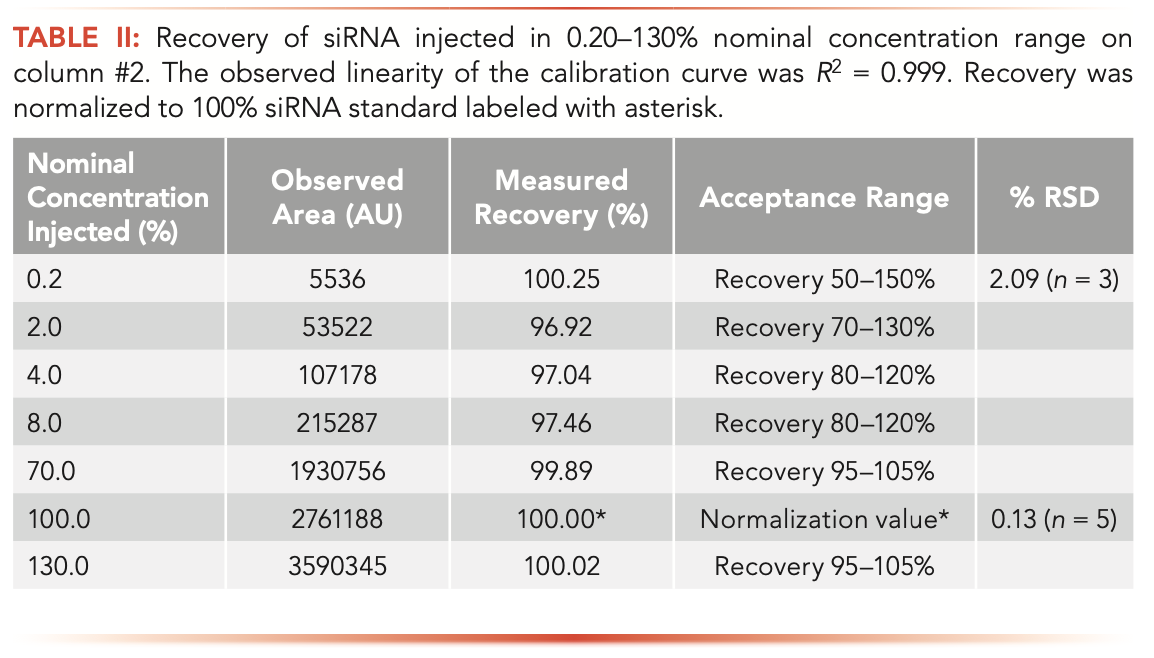
It has been reported that the loss of sample recovery is most apparent at low concentrations (17). This occurrence has a direct impact on the quality of the quantitation results. A loss of dynamic range and linearity of calibration curves was noted in nucleic acid analysis (18,19). Good sample recovery is paramount for constructing valid calibration curves. Table II shows siRNA recovery in the 0.20–130% nominal concentration range (Duplex C) observed in LC analysis with column #2. A minor sample loss is still observed at the low end of concentration range; however, the recovery, repeatability, and linearity (R2 = 0.999) meet the qualification parameters set in our laboratory. Based on our findings, we selected column #2 for siRNA analysis.
Non-Denaturing LC Method for Duplex siRNA Analysis
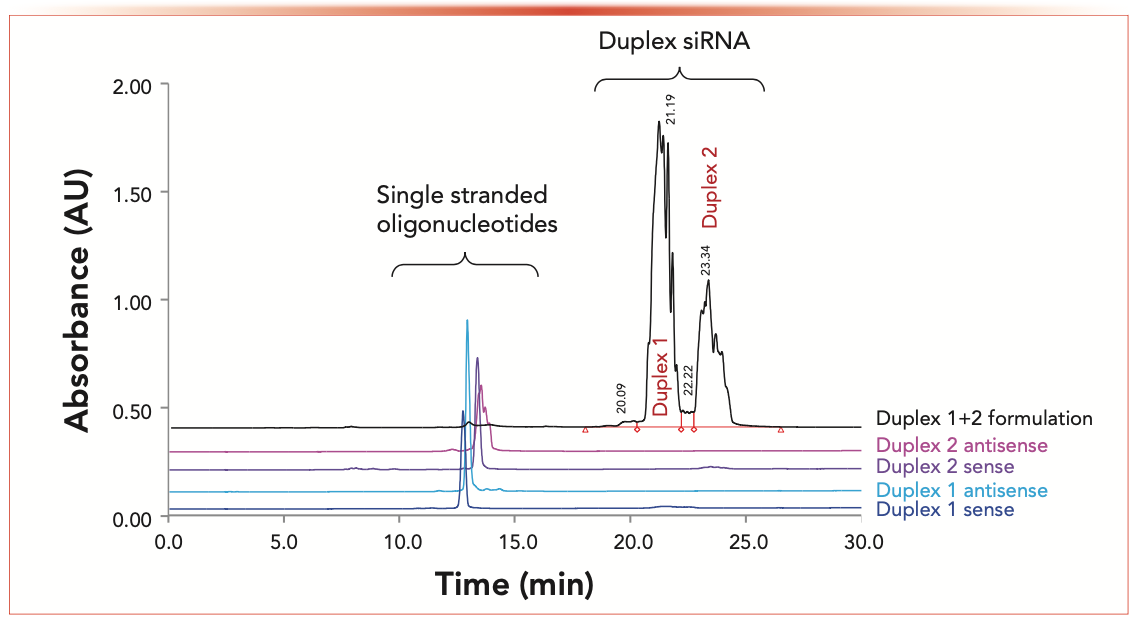
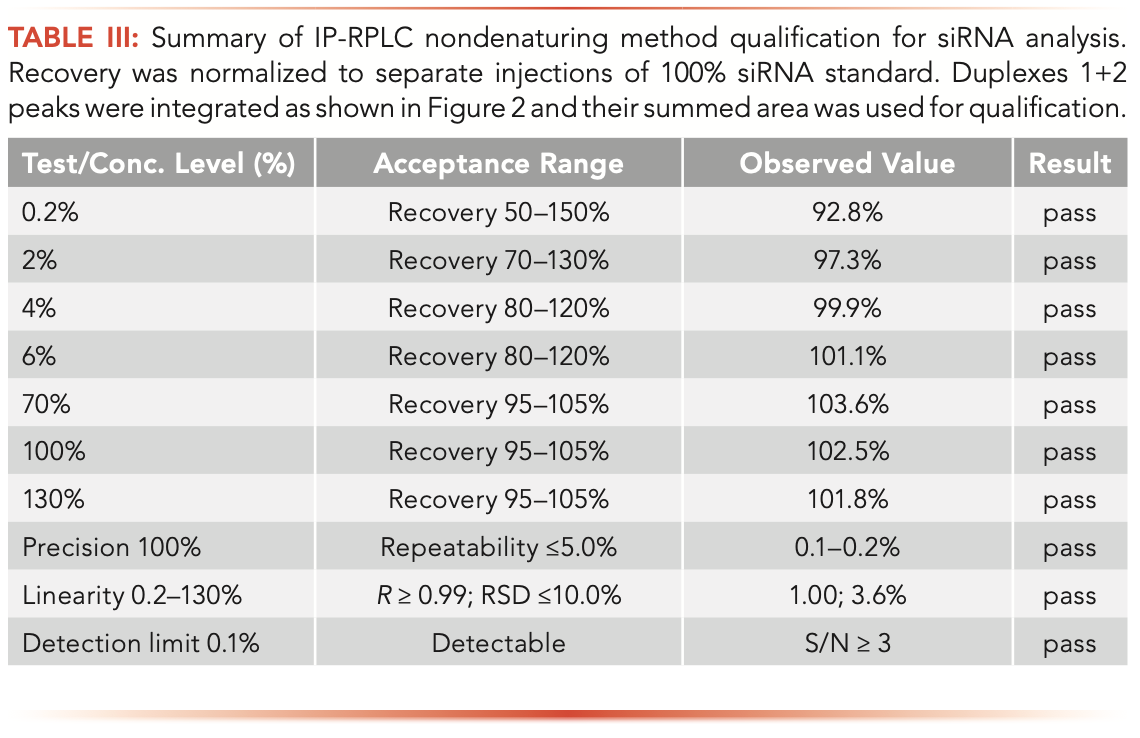
Column #2 was further applied for analyzing intact siRNA duplexes. Figure 2 illustrates the analysis of a drug formulation containing two siRNA duplexes under non-denaturing LC conditions. The Duplex 1 and 2 siRNA species are more strongly retained in IP-RPLC compared to oligonucleotides (10). Single- stranded species are eluted between 12–13 min, well resolved from the duplexes (Figure 2). The method enabled the separation of two siRNA duplexes that were eluted at 21.19 and 23.34 min. One may notice that Duplexes 1 and 2 (black chromatogram in Figure 2) and single-stranded species (for example, the antisense strand of Duplex 2) are resolved into multiple peaks, which is a consequence of thioation of selected internucleotide linkages. The resulting diastereoisomers can be chromatographically resolved (24–26); this results in “band broadening” and complicates the LC analysis. Despite partial separation of siRNA duplexes into isomers, the developed non-denaturing LC method enables quantification of duplexes and their single-stranded impurities. Table III summarizes the method qualification. All of the method qualification criteria, such as repeatability, accuracy, linearity, quantitation, and detection limits, were met.
FIGURE 2: Separation of single-stranded RNA oligonucleotides from siRNA duplexes in a nondenaturing IP-RPLC method.
siRNA Carryover Study
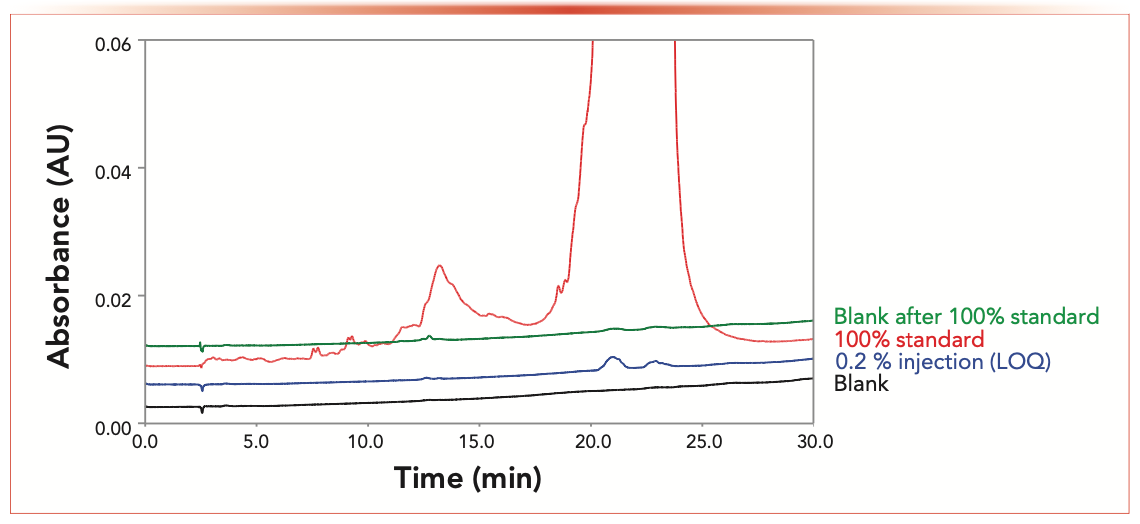
An underappreciated aspect of nucleic acid LC analysis is the potential for higher than usual analyte carryover (15,17). This carryover is primarily caused by sample adsorption on column metal frits and to a lesser degree because of nonspecific adsorption on injector or sorbent surfaces (21,23,27,28). We evaluated the siRNA carryover for column #2 using the non-denaturing siRNA method. Figure 3 compares a blank chromatogram, devoid of any significant system peaks, with 0.20% limit of quantification (LOQ) standards of Duplexes 1 and 2, followed by an injection of a 100% sample standard and a subsequent blank injection. Minimal siRNA carryover was observed in post 100% standard blank injection. In comparison, typical carryover on a stainless-steel column detected in a similar experiment was at levels matching or exceeding the 0.2% LOQ threshold (data is not shown in Figure 3). The elevated carryover is likely caused by siRNA adsorption on column hardware followed by sample desorption and concentration on column during the equilibration prior to next injection (17,18). We conclude that column #2 with the hardware modified by HPS is our preferred choice for siRNA analysis.
FIGURE 3: IP-RPLC carryover study for the nondenaturing LC method.
Conclusions
Denaturing and non-denaturing IP-RPLC methods were developed for analysis of single-stranded and duplex siRNA. The denaturing method permitted separation of siRNA oligonucleotides and quantitation of their synthetic impurities. The non-denaturing method was used for quantification of excess of single-stranded species in the siRNA duplex formulations. Both methods utilized MS-compatible mobile phases; the peak identities were verified by the mass spectra. We demonstrated that column #2 is suitable for robust quantitation of siRNA duplexes without significant carryover. The methods afforded high confidence of analysis, and fulfilled the qualification parameters of recovery, repeatability, and linearity. We could conclude that the HPS-modified column hardware is a preferred option for nucleic acid analysis.
References
(1) Lundin, K. E.; Gissberg, O.; Smith, C. I. Oligonucleotide Therapies: The Past and the Present. Hum. Gene Ther. 2015, 26 (8), 475–485. DOI: 10.1089/hum.2015.070
(2) Khvorova, A.; Watts, J. K. The Chemical Evolution of Oligonucleotide Therapies of Clinical Utility. Nat. Biotechnol. 2017, 35 (3), 238–248. DOI: 10.1038/nbt.3765
(3) Gilar, M. Analysis and Purification of Synthetic Oligonucleotides by Reversed-Phase High-Performance Liquid Chromatography with Photodiode Array and Mass Spectrometry Detection. Anal. Biochem. 2001, 298 (2), 196–206. DOI: 10.1006/abio.2001.5386
(4) Gilar, M.; Fountain, K. J.; Budman, Y.; Holyoke, J. L.; Davoudi, H.; Gebler, J. C. Characterization of Therapeutic Oligonucleotides Using Liquid Chromatography with Online Mass Spectrometry Detection. Oligonucleotides 2003, 13 (4), 229–243. DOI: 10.1089/154545703322460612
(5) Apffel, A.; Chakel, J. A.; Fischer, S.; Lichtenwalter, K.; Hancock, W. S. Analysis of Oligonucleotides by HPLC-Electrospray Ionization Mass Spectrometry. Anal. Chem. 1997, 69, 1320–1325.
(6) Gong, L.; McCullagh, J. S. Comparing Ion- Pairing Reagents and Sample Dissolution Solvents for Ion-Pairing Reversed-Phase Liquid Chromatography/Electrospray Ionization Mass Spectrometry Analysis of Oligonucleotides. Rapid Commun. Mass Spectrom. 2014, 28 (4), 339–350. DOI: 10.1002/rcm.6773
(7) Li, N.; El Zahar, N. M.; Saad, J. G.; van der Hage, E. R. E.; Bartlett, M. G. Alkylamine Ion-Pairing Reagents and the Chromatographic Separation of Oligonucleotides. J. Chromatogr. A 2018, 1580, 110–119. DOI: 10.1016/j.chroma.2018.10.040
(8) Studzińska, S.; Rola, R.; Buszewski, B. The Impact of Ion-Pairing Reagents on the Selectivity and Sensitivity in the Analysis of Modified Oligonucleotides in Serum Samples by Liquid Chromatography Coupled with Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2017, 138, 146–152. DOI: 10.1016/j.jpba.2017.02.014
(9) Donegan, M.; Nguyen, J. M.; Gilar, M. Effect of Ion-Pairing Reagent Hydrophobicity on Liquid Chromatography and Mass Spectrometry Analysis of Oligonucleotides. J. Chromatogr. A 2022, 1666, 462860. DOI: 10.1016/j.chroma.2022.462860
(10) McCarthy, S. M.; Gilar, M.; Gebler, J. Reversed-Phase Ion-Pair Liquid Chromatography Analysis and Purification of Small Interfering RNA. Anal. Biochem. 2009, 390 (2),181–188. DOI: 10.1016/j.ab.2009.03.042
(11) McCarthy, S. M.; Gilar, M. Waters Application Note 720003361EN (2010).
(12) Levin, D. S.; Shepperd, B. T.; Gruenloh, C. J. Combining Ion Pairing Agents for Enhanced Analysis of Oligonucleotide Therapeutics by Reversed Phase-Ion Pairing Ultra Performance Liquid Chromatography (UPLC). J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879 (19), 1587–1595. DOI: 10.1016/j.jchromb.2011.03.051
(13) Ivleva, V. B. Waters Application Note 720002412EN (2008).
(14) Ivleva, V.B.; Yu, Y.Q.; Gilar, M. Ultra-Performance Liquid Chromatography/Tandem Mass Spectrometry (UPLC/MS/MS) and UPLC/MSE Analysis of RNA Oligonucleotides. Rapid Commun. Mass Spectrom. 2010, 24 (17), 2631–2640. DOI: 10.1002/rcm.4683
(15) Lauber, M. A.; Walter, T. H.; Gilar, M.; DeLano, D.; Boissel, C.; Smith, K.; Birdsall, R. E.; Rainville, P.; Belanger, J.; Wyndham, K. White Paper, Waters 720006930EN (2021).
(16) DeLano, M.; Walter, T. H.; Lauber, M. A.; Gilar, M.; Jung, M. C.; Nguyen, J. M.; Boissel, C.; Patel, A. V.; Bates-Harrison, A.; Wyndham, K. D. Using Hybrid Organic–Inorganic Surface Technology to Mitigate Analyte Interactions with Metal Surfaces in UHPLC. Anal. Chem. 2021, 93 (14), 5773–5781. DOI: 10.1021/acs.analchem.0c05203
(17) Gilar, M.; DeLano, M.; Gritti, F. Mitigation of Analyte Loss on Metal Surfaces in Liquid Chromatography. J. Chromatogr. A 2021, 1650, 462247. DOI: 10.1016/j.chroma.2021.462247
(18) J.M. Nguyen, M. Gilar, B. Koshel, M. Donegan, J. MacLean, Z. Li, M. A. Lauber, Assessing the Impact of Nonspecific Binding on Oligonucleotide Bioanalysis. Bioanalysis 2021, 13 (16), 1233-1244. DOI: 10.4155/bio-2021-0115
(19) Suarez Marina, I.; Verluyten, W.; Dejaegere, E.; Napoletano, L.; Boon, J.-P.; Hellings, M.; Gilar, M. Waters Application Note 720007362 (2021).
(20) Yogendrarajah, P.; Verluyten, W.; Dejaegere, E.; Napoletano, L.; Boon, J.-P.; Hellings, M.; Gilar, M. Waters Application Note 720007361 (2021).
(21) Tuytten, R.; Lemière, F.; Witters, E.; Van Dongen, W.; Slegers, H.; Newton, R. P.; Van Onckelen, H.; Esmans, E. L. Stainless Steel Electrospray Probe: A Dead End for Phosphorylated Organic Compounds? J. Chromatogr. A 2006, 1104 (1–2), 209–221. DOI: 10.1016/j.chroma.2005.12.004
(22) Nagayasu, T.; Yoshioka, C.; Imamura, K.; Nakanishi, K. Effects of Carboxyl Groups on the Adsorption Behavior of Low-Molecular-Weight Substances on a Stainless Steel Surface. J. Colloid Interface Sci. 2004, 279 (2), 296–306. DOI: 10.1016/j.jcis.2004.06.081
(23) Guimaraes, G. J.; Sutton, J. M.; Gilar, M.; Donegan, M.; Bartlett, M. G. Impact of Nonspecific Adsorption to Metal Surfaces in Ion Pair-RP LC-MS Impurity Analysis of Oligonucleotides. J. Pharm. Biomed. Anal. 2002, 208, 114439. DOI: 10.1016/j.jpba.2021.114439
(24) Li, L.; Leone, T.; Foley, J. P.; Welch, C. J.; Separation of Small Interfering RNA Stereoisomers Using Reversed-Phase Ion-Pairing Chromatography. J. Chromatogr. A 2017, 1500, 84–88. DOI: 10.1016/j.chroma.2017.04.008
(25) Gilar, M.; Belenky, A.; Budman, Y.; Smisek, D. L.; Cohen, A. S. Impact of 3’-exonuclease Stereoselectivity on the Kinetics of Phosphorothioate Oligonucleotide Metabolism. Antisense Nucleic Acid Drug Dev. 1998, 8 (1), 35–42. DOI: 10.1089/oli.1.1998.8.35
(26) Stec, W. J.; Zon, G.; Uznanski, B. Reversed-Phase High-Performance Liquid Chromatographic Separation of Diastereomeric Phosphorothioate Analogues of Oligo-Deoxyribonucleotide and Other Backbone-modified Congeners of DNA. J. Chromatogr. 1985, 326, 263–280.
(27) Birdsall, R. E.; Kellett, J.; Ippoliti, S.; Ranbaduge, N.; Lauber, M. A.; Yu, Y. Q.; Chen, W. Reducing Metal-ion Mediated Adsorption of Acidic Peptides in RPLC-based Assays Using Hybrid Silica Chromatographic Surfaces. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1179, 122700. DOI: 10.1016/j.jchromb.2021.122700
(28) Birdsall, R. E.; Kellett, J.; Yu, Y. Q.; Chen, W. Application of Mobile Phase Additives to Reduce Metal-ion Mediated Adsorption of Non-Phosphorylated Peptides in RPLC/MS-based Assays. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2019, 1126–1127, 121773. DOI: 10.1016/j.jchromb.2019.121773
Pratheeba Yogendrarajah, Irene Suarez Marina, Willy Verluyten, Jean-Paul Boon, and Mario Hellings are with Janssen Pharmaceutical Companies of Johnson & Johnson, in Beerse, Belgium. Evelien Dejaegere, Leslie Napoletano, and Martin Gilar are with Waters Corporation, in Milford, Massachusetts. Direct correspondence to: martin_gillar@waters.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.













