Analysis of 14 Cannabinoids
In order to evaluate and test the robustness of cannabinoid analysis using the miniLC, a method was chosen from the literature and compared to data from the miniLC system. Comparison of retention time of standards to published values indicates the miniLC provides an excellent alternative with comparable results to a more expensive, complicated liquid chromatography system.
With growth in the food, health, and personal care market, understanding the presence and relative amounts of cannabinoids in a variety of samples is becoming increasingly important. Making this analysis accessible to a wide variety of testing laboratories and manufacturers will serve to enhance reliability, access, and the relative amount of information available. Herein is described a simple first step toward making cannabinoid analysis available for everyone.
Materials and Methods
A Lucidity miniLC LC-UV with Restek Ultra C18 column (150 mm ×
4.6 mm × 5 µm) was used for characterization of the cannabinoid mixture with the following method parameters:
- Column Temp: 40 °C
- Flow Rate: 2.0 mL/min
- Injection Volume: 10 µL
- Wavelength: 230 nm
- Mobile Phase A: Water + 0.1% Formic Acid + 5 mM Ammonium Formate
- Mobile Phase B: Acetonitrile
- Isocratic Conditions 70% B
A mixture of 14 cannabinoids was made, using 14 individual Cerilliant cannabinoid standards, obtained from Millipore Sigma. Each of the standards was 1.0 mg/mL of compound in either methanol or acetonitrile, as outlined below. These standards were:
- CBDV-A: C-152-1ML, in MeCN
- CBDV: C-140-1ML, in MeOH
- CBD-A: C-144-1ML, in MeCN
- CBG-A: C-142, in MeCN
- CBG: C-141-1ML, in MeOH
- THCV: T-094, in MeOH
- THCV-A: T-111, in MeCN
- CBN: C-046-1ML, in MeOH
- Δ9-THC: T-005, in MeOH
- Δ8-THC: T-032, in MeOH
- CBL: C-154-1ML, in MeCN
- THC-A: T-093-1ML, in MeCN
- CBC: C-143, in MeOH
- CBC-A: C-150-1ML, in MeCN
They were combined into a single mixture with a concentration of 71.4 µg/mL (roughly 91 ppm) of each compound. An additional 2 dilutions were created based on this mixture of 39.3 µg/mL (approximately 50 ppm) and 7.85 µg/mL (approximately 10 ppm) of each compound.
With a 10 µL injection volume this correlates to the following three concentrations on column:
- Concentration 1 714 ng on column
- Concentration 2 393 ng on column
- Concentration 3 78.5 ng on column
Results
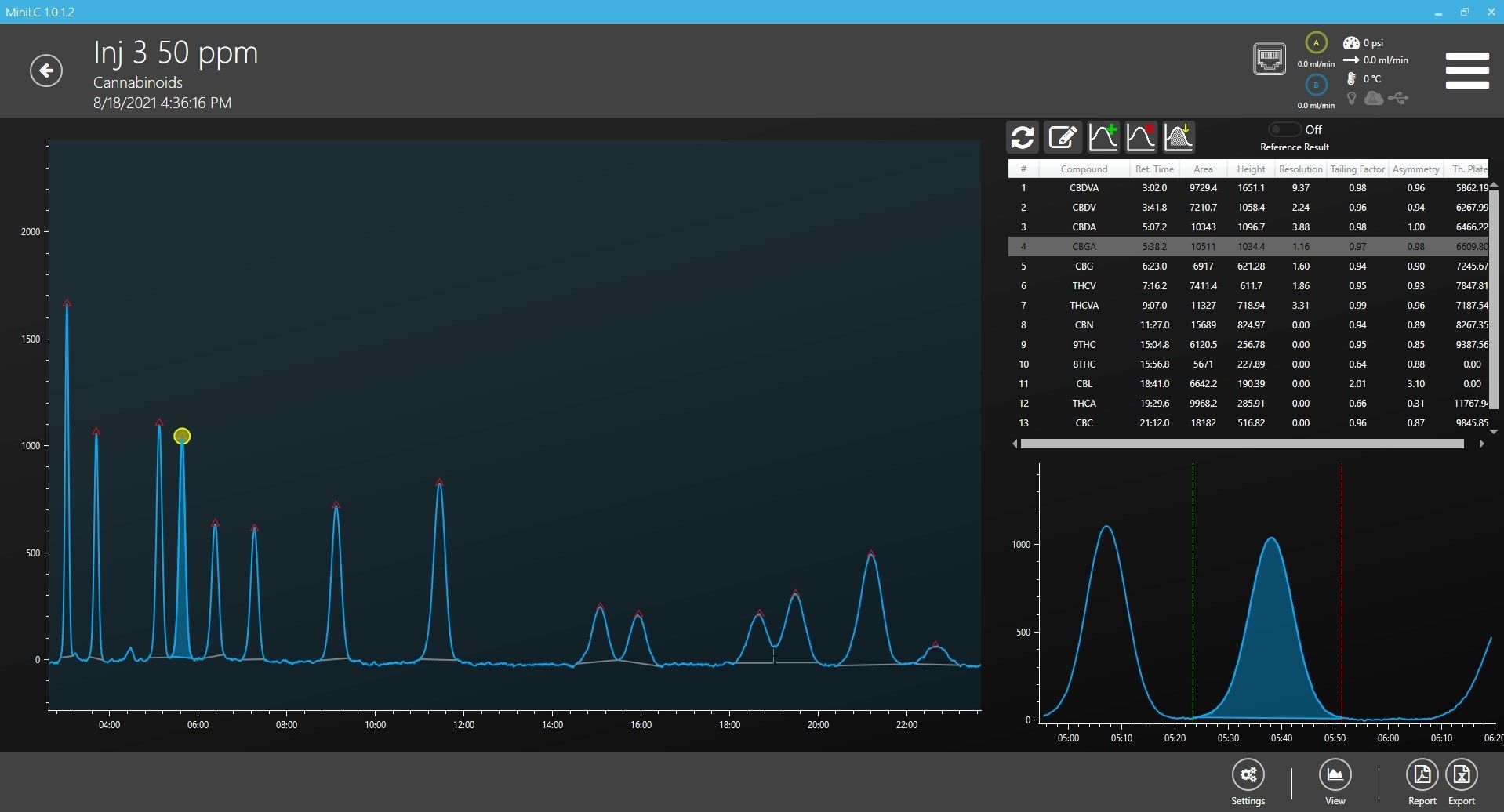
First, we injected each concentration of standard 3 times in a row to test repeatability and linearity of calibration curves over this range of concentrations. Figure 1 shows an overlay of the 3 injections from the middle concentration (39.3 µg/mL or 393 ng on column). Figure 2 shows one of the 39.3 µg/mL injections with peaks identified and the peak list on right.
Figure 1: Three injections of 39.3 µg/mL overlaid
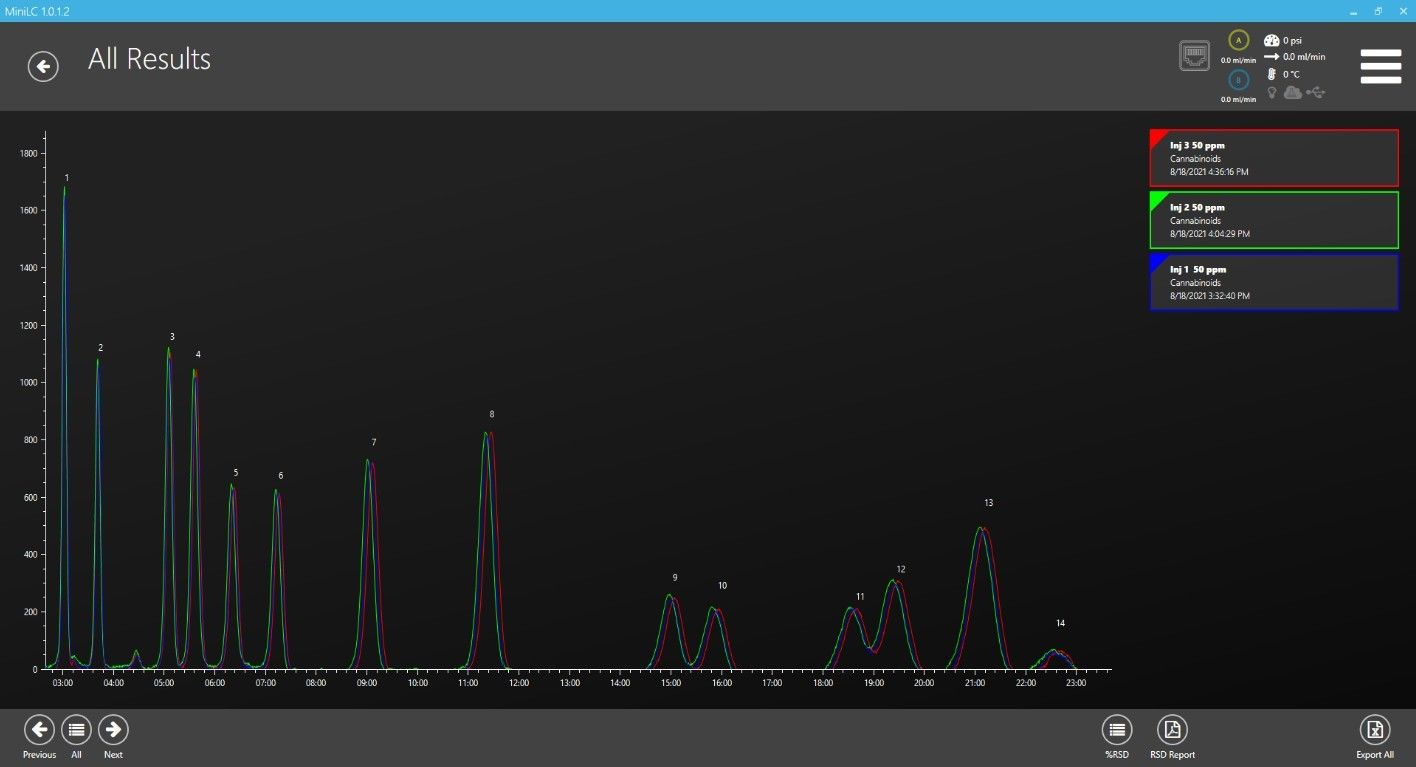
Figure 2: One injection of 39.3 µg/mL (393 ng on column) with peak list
We also observed good RSDs for the 3 injections at each concentration and good R2 values for 3 of the compounds that were chosen at random and used to create calibration curves.
The RSDs for the 3 injections of the middle concentration (39.3 µg/mL, 393 ng on column) were as follows:
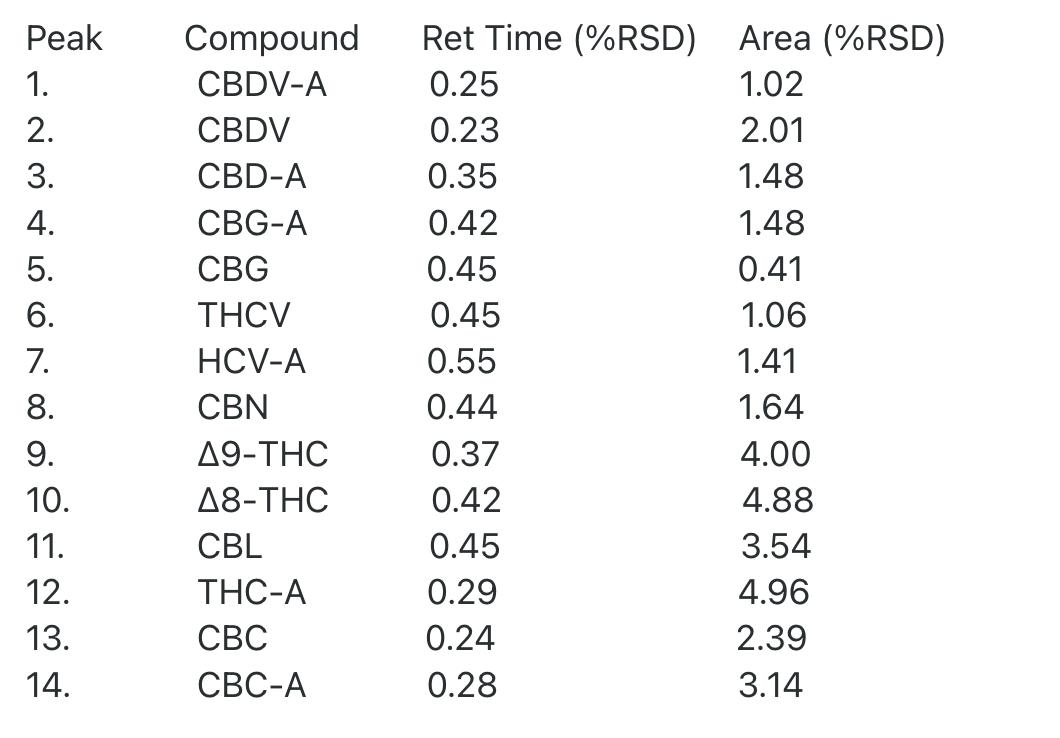
All RSDs for retention time were less than 1% and all RSDs for peak areas were less than 5% with many being under 2%.
Conclusions
This was a quick study on cannabinoids in the miniLC that compared the overall quality of chromatograms achieved, the repeatability over multiple injections, and the linearity of the system across multiple concentrations. Overall, the results were positive, demonstrating the effectiveness of the miniLC for analyzing and quantifying cannabinoids.
CEM Corporation
3100 Smith Farm Road, Matthews, NC 28104
Tel. (800) 726-3331



