Ambient Ionization MS Analysis of Swabs Used for Explosives Detection
Special Issues
An analysis of the capabilities of direct analysis in real time (DART) and desorption electrospray ionization (DESI) sources for the analysis of contaminated fabrics
This article describes the capabilities of direct analysis in real time (DART) and desorption electrospray ionization (DESI) sources for the analysis of contaminated fabrics. The effect of key parameters including the source conditions, the mesh size of different fabrics, and the use of additives to enhance detection limits is investigated and the suitability of both sources is discussed. The successful analysis of real cotton fabric samples illustrates the efficiency of ambient ionization mass spectrometry, which tends to change the instrumental arsenal of the expert laboratories.
Rapid analysis of explosive traces from solid samples (soils, debris, or clothing) is a challenging task for forensic investigations, homeland security, or environmental remediation. Small surfaces can be investigated as such, but larger ones require sampling methods. The use of wipes for collection of explosives is therefore common practice. Examples of applications include hand sampling for exposure control, airport luggage screening ,or inspection of suspicious installations in response to an allegation of use. Subsequent analysis of the wipes is traditionally performed by a solvent extraction step, eventually accompanied by a preconcentration step, and finally followed by gas chromatography–mass spectrometry (GC–MS) or liquid chromatography–mass spectrometry (LC–MS) analysis (1). The total sample analysis time can be greatly increased by these laborious sample preparation steps. Moreover, supplementary sample manipulations can lead to higher cross-contamination risks.
The introduction of desorption electrospray ionization (DESI [Prosolia]) in 2004 (2) and direct analysis in real time (DART [IonSense]) in 2005 (3) paved the way for a new field of MS for direct analysis: ambient MS. To date, more than 30 ambient ionization sources have been developed and described in the literature (4). The main innovation lies in the fact that these sources allow direct analysis of compounds from a wide variety of surfaces, with little or no sample pretreatment. This is achieved by directing a stream of ionizing fluid (liquid or gas) onto the sample surface from which compounds are withdrawn and ionized before being transported though air into the mass analyzer. In every scenario, charged aggregates are formed, the size of which depends upon desolvation conditions. Another interesting feature is the minimally invasive character of most ambient ionization techniques. Depending on the ionization process involved, DESI, DART, and all the related techniques thus allow direct and rapid (within a few seconds) analysis of almost any kind of sample surfaces (glass, plastic, paper, or vegetal and biological tissues), under open-air conditions. Ambient ionization has already demonstrated its potential for various applications like control of food contaminants (pesticides, herbicides, fungicides, and packaging migrants), analysis of pharmaceuticals, tissue imaging, metabolite identification in biological fluid samples, or forensic investigations (explosives, drugs of abuse, and inks) (4).
Taking advantage of ambient MS, both the DESI–MS and the DART–MS methods have been explored for rapid analysis of wipes used for collection of explosives from surfaces. They have been applied to the sensitive detection of 1,3,5 trinitro 1,3,5 triazine (RDX) traces deposited on different fabrics. After optimization of the operating parameters, the performance and the suitability of both these ambient ionization sources are discussed.
Experimental
Chemicals
RDX (1 mg/mL in 1:1 methanol–acetonitrile) was purchased from AccuStandard Europe. HPLC-grade methanol was obtained from VWR and water was purified with a Millipore MilliQ-10 system. Standard solutions of RDX were obtained by dilution in appropriate proportion of solvent. In specific experiments, ammonium chloride (Suprapur, Merck) was added to favor the formation of chloride adduct anions. All solutions were kept in the dark and refrigerated.
Sample Preparation
The wipes used for this study consisted of little pieces of fabrics (4 × 7 cm). Four fabrics (starched cotton [a], two polyesters [b and c], and pure cotton [d]) were selected based on their different respective mesh size (Figure 1) and chemical composition.

Figure 1: Magnified views of the studied fabrics: (a) starched cotton, (b) polyester, (c) polyester, (d) pure cotton.
Fabrics were spiked with different quantities of RDX and used as sample surfaces for DESI-MS and DART-MS experiments. RDX standard solutions were diluted in 1:1 water–methanol to concentrations ranging from 0.1 mg/L to 900 mg/L. In the case of experiments using the additive, ammonium chloride was added to each solution so that the final chloride concentration reached 1 mM. Then 1 μL was spotted onto the fabrics so that deposited mass ranged from 0.1 to 900 ng. Physical transfer was the other deposition method tested. The range 0.1–900 ng was deposited on a glass slide and allowed to dry. The glass slide was then wiped down with the fabric and the latter was directly analyzed by DESI-MS or DART-MS.
MS Apparatus and Conditions
All experiments were performed in the negative ion mode using an LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific) tuned for optimum detection of the ion of interest. The 150–500 Th mass range was monitored in total ion current (TIC) mode. The transfer capillary temperature was set to 250 °C, the transfer capillary voltage to -50 V, and the tube lens voltage to 100 V. The resolving power of the mass spectrometer was set to 30,000 and external mass calibration was performed every two days. Xcalibur (2.0.7 SP1) software was used for data acquisition and post-processing.
All DESI experiments were carried out using an OmniSpray 2D ion source from Prosolia Inc. Fabric samples spiked with RDX were attached to a glass slide with double-face adhesive tape at both ends. Optimized experimental parameters are summarized in Table I.
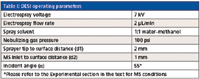
Table I: DESI operating parameters
DART experiments were performed using a DART-SVP ion source (IonSense). When the DART ion source was operated in reflection mode, samples were fixed as for DESI experiments. DART was also operated in transmission mode. In that case, fabrics were fixed on a frame and placed at 0.7 cm and perpendicularly to the evaporating–ionizing gas beam. In this study, helium was used for operations and its pressure was set to 80 psi. Nitrogen was used in the standby mode. Tested gas temperatures ranged from 150 to 450 °C.
Results and Discussion
The Importance of High Resolution
In the absence of a separation method, reliable identification of chemicals by MS can be obtained by tandem MS or high-resolution MS (HRMS). However, to achieve global analysis of complex samples, HRMS seems to be more appropriate. Ambient ionization sources were thus combined with HRMS to limit the number of candidate elemental compositions to a single confident assignment. If sufficient resolving power is used, it is possible to distinguish the target compound from any possible interference and, thus, to limit the number of false-positive or false-negative results. In the specific case of explosive analysis, the deprotonated TATB molecule (C6H5O6N6, calculated m/z 257.02761) and the chloride adduct anion of RDX (C3H6O6N635 Cl, calculated m/z 257.00428) are isobaric compounds and can easily be confused. A minimum resolving power of 30,000 (full width at half maximum [FWHM]) is necessary to resolve their respective peaks completely (Figure 2).
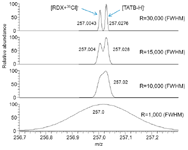
Figure 2: Effect of the instrumental resolving power on separation of isobaric ions (C6H5O6N6 and C3H6O6N635Cl).
Method Development and Operating Parameters Optimization
DESI
DESI is an ambient ionization method based on the impact of charged, high-velocity solvent droplets generated from a pneumatically assisted electrospray emitter onto a surface. It is believed that primary microdroplets impacting the surface form a thin solvent layer where analytes can migrate according to a solid–liquid extraction process. Subsequent microdroplets splashing into this solvent layer release secondary microdroplets containing the dissolved analytes (Figure 3).
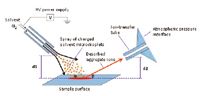
Figure 3: Scheme of the DESI-MS system. Adapted from reference 2.
DESI performance is highly dependent on a very large number of experimental parameters. Geometrical parameters as well as surface, solvent, and spray parameters have strong effects on ionization process and ion collection efficiency (5). A meticulous optimization of experimental parameters is therefore necessary. Values set for DESI experiments are summarized in Table I.
DART
Reactive species responsible for DART ionization are metastable atoms or molecules of inert gas (typically helium or nitrogen), generated by electrical discharge. They subsequently react in the gas phase by Penning ionization with ambient oxygen and water to produce ions that react with polar or nonpolar analytes as occurs in atmospheric-pressure chemical ionization (APCI).
The effect of gas temperature on analyte ion abundance was evaluated. Figure 4 shows that raising the temperature leads to signal increase. However, the fabric begins to degrade at 400 °C leading to new abundant peaks. As a consequence, DART gas temperature was finally set to 350 °C. Regarding geometric optimization, DART can be operated either in reflection mode or in transmission mode. Results obtained in reflection mode were not reproducible and the signal fluctuated significantly from one experiment to the next (coefficient of variation [CV] > 130%). Transmission mode (TM-DART) was therefore preferred and used unless stated otherwise.
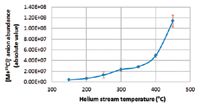
Figure 4: Effect of the DART gas temperature on RDX chloride adduct anion abundance. Standard deviations are depicted in red.
Effect of Deposition Method and Fabric Features
Two RDX deposition methods were used to prepare the fabric samples. The first one involves solution deposition of 1 μL of standard solution directly onto the surface. In the case of DART or DESI experiments, ion abundance was very dependent on the fabric's physical and chemical properties. Openwork fabrics (Figures 1a, 1b, and 1c) allowed molecules contained in the deposited droplet to remain accessible for the charged gas or droplet beam, and thus they were efficiently ionized or desorbed. However, little to no signal was obtained in the case of the hydrophilic fabric shown in Figure 1d. It seems that after solution deposition on hydrophilic and very dense fabrics, molecules are deeply absorbed, disturbing their release. This observation is all the more pronounced when the fabric is dense. To avoid this drawback and mimic real wipes used for surface investigation, fabrics were also contaminated by physical transfer. Identical RDX quantities were deposited on a glass slide, allowed to dry, wiped down with the fabric, and then directly analyzed. Signal intensities for physical transfer on the fabrics shown in Figures 1a–1c were lower than those for solution deposition (Figure 5). It is expected that during physical transfer, explosive particles are collected on a larger area (fingertip contact area equal to about 2.5 cm2) than that of the solvent droplet (>0.5 cm2), resulting in lower signal intensities. Moreover, it has to be noted that signal intensity reproducibility after physical transfer is rather low (relative standard deviation [RSD] ≈ 40–60%) compared with solution deposition (RSD < 30%).
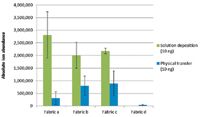
Figure 5: Effect of the deposition method on [RDX+Cl]â ion abundance for the four studied fabrics. Note that results for fabric d were obtained in reflection mode DART.
In the special case of the pure cotton fabric (Figure 1d), no signal could be obtained in transmission mode DART whether after solution deposition or physical transfer. It seems that the textile is too opaque and that no helium can possibly get through. TM-DART suitability for fabric analysis is thus limited by the possibility for the gas beam to be efficiently transmitted through the sample. Results displayed for the pure cotton fabric in Figure 5 were therefore obtained in reflection mode DART. In that case, higher signal intensities were obtained for fabric d after physical transfer probably because analyte particles remained on the surface of the sample fibers and were not adsorbed as occurs when solution deposition is used.
Additional studies were performed to determine the explosive amount transferred during swabbing. Explosive remains on the glass slide were extracted by the solvent and quantitatively analyzed using LC–MS. Results suggest that physical transfer yield generally exceeds 90%.
Effect of Additive Use
In DESI and DART sources, RDX is mainly detected by the attachment of an anion, although [RDX-H]– can appear on mass spectra in very low abundance (because of low gas phase methylene acidity). Characteristic adduct ions include [RDX+HCOO]–, [RDX+CH3COO]–, [RDX+Cl]–, or [RDX+NO2]– produced in competition and arising from the sample environment. Without additive use, these adduct anions are formed indifferently. To control the ionization process and favor one adduct anion, it is a common practice to introduce an additive either in the sample or in the DESI spray solvent. For this purpose, ammonium chloride was added to the RDX standard solution to enhance the signal. Chloride salt was chosen because of its high electron affinity. Moreover, the chloride adduct is characterized by its specific isotopic pattern (M and M+2 present in a 3:1 ratio) giving additional indication for unequivocal compound identification, in addition to accurate mass measurements. Different ammonium chloride concentrations were tested and the optimal concentration was found to be 1 mM, resulting in great enhancement of [RDX+Cl]– adduct ion abundance. In these conditions, signal increase was about 60–80% and detection limits as low as 0.1 ng have been obtained with the DART. Figure 6 shows DART extracted ion chromatograms (EIC) for [RDX+35 Cl]– (m/z 257.0043 with a 10 ppm mass tolerance) for 10 ng of RDX deposited with and without the addition of 1 mM ammonium chloride. The corresponding mass spectrum is displayed in the inlay.

Figure 6: Overlay extracted ion chromatograms (m/z 257.043). In red: 10 ng deposited without any chloride addition. In blue: 10 ng deposited with 1 mM ammonium chloride.
Comparison Between DESI and DART Ionization
To compare DESI and DART performances, a concentration range (0.1–900 ng) of RDX standard solution in water–methanol with 1 mM ammonium chloride was deposited in triplicate on the fabric shown in Figure 1c. It appears that in the same conditions (concentration, fabric, chloride ion generation, and deposition method), DART is about 100 times more sensitive than DESI for analysis of wipes used for explosives sampling. The limit of detection (LOD) obtained in DESI is about 10 ng whereas quantities as low as 100 pg deposited on the fabric can be detected in DART. However, it should be noted that the lower suitability of DESI in this particular case does not arise from the ionization process itself. In fact, electrospray mechanisms prevail in DESI and the former is known to be well suited for nitramines analysis. Moreover, DESI works perfectly well using the Omni Slide Hydrophobic Array (Prosolia, Inc.). Lower ionization efficiency most likely comes from adsorption of the RDX molecules on the textile fibers and absorption of the DESI spray solvent inside the fabric. In these conditions, local extraction of the analytes and their subsequent release by impacting droplet is difficult.
In DART, the ionization process is akin to APCI and takes place in the gas phase. Even if RDX vapor pressure is low (4.6 × 10-9 Torr at 25 °C), it is sufficient for DART-MS analysis. Gas heating also contributes to better analyte evaporation.
Moreover, it is necessary to point out that we didn't try transmission mode DESI. This operating mode has been previously tested for the analysis of insecticide-treated bed nets and showed promising results with open mesh structure nets (6).
Conclusion
We have shown that DART seems more suitable than DESI for trace analysis of RDX on fabrics when used in the transmission mode. This is mainly due to the ionization mechanisms involved. DART is a gas-phase ionization (Penning ionization) technique. In the case of RDX, its vapor pressure is low but sufficient for DART-MS analysis. On the contrary, the use of the DESI source is less suited for fabric analysis because the spray solvent is absorbed in the textile fibers. The release of offspring droplets by the splash process involved in desorption is thus compromised. However, TM-DART ionization efficiency is limited by the mesh characteristics. On one hand, openwork fabrics allow the evaporating- ionizing gas beam to pass through the sample, resulting in an increased sensitivity. On the other hand, completely opaque fabrics prevent ion transmission into the mass analyzer, resulting in the absence of the signal of interest. In optimized conditions and favored by chloride addition, LOD in the low nanograms range can be obtained with DESI while DART shows LOD in the picogram range.
Further studies are currently being carried out for a better and more extensive understanding of the role played by the fabric features (mesh size and chemical composition) and will be extended to other explosives.
References
(1) J. Yinon and S. Zitrin, in Modern Methods and Applications in Analysis of Explosives, (John Wiley & Sons Ltd, Chichester, UK, 1993), pp. 163–209.
(2) Z. Takáts, J.M. Wiseman, B. Gologan, and R.G. Cooks, Science 306, 471–473 (2004).
(3) R.B. Cody, J.A. Laramee, and H.D. Durst, Anal. Chem. 77, 2297–2302 (2005).
(4) G.A. Harris, A.S. Galhena, and F.M. Fernández, Anal. Chem. 83, 4508–4538 (2011).
(5) Z. Takáts, J.M. Wiseman, and R.G. Cooks, J. Mass Spectrom. 40, 1261–1275 (2005).
(6) J.J. Pérez, G.A. Harris, J.E. Chipuk, J.S. Brodbelt, M.D. Green, C.Y. Hampton, and F.M. Fernández, Analyst 135, 712–719 (2010).
Cécile Hubert and Dr. Xavier Machuron-Mandard are with CEA, DAM, DIF, F-91297 in Arpajon, France. Professor Jean-Claude Tabet is with the Institut Parisien de Chimie Moléculaire (Chimie Structurale Organique et Biologique) at the Université Pierre et Marie Curie in Paris, France.
Direct correspondence to: xavier.machuron-mandard@cea.fr

Characterizing Plant Polysaccharides Using Size-Exclusion Chromatography
April 4th 2025With green chemistry becoming more standardized, Leena Pitkänen of Aalto University analyzed how useful size-exclusion chromatography (SEC) and asymmetric flow field-flow fractionation (AF4) could be in characterizing plant polysaccharides.
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).








