Advances in Sample Preparation for Biological Fluids
Special Issues
Sample preparation techniques in bioanalysis are multistep, time-consuming, and labour-intensive procedures that can take up 60–80% of the total analysis time. Sample preparation is often the limiting step of fast bioanalysis and the most error-prone part of the analytical method. There is currently a focus on improving the sample preparation process by shortening sample preparation time, cutting the cost of analysis, decreasing sample volume and solvent consumption, reducing the number of sample preparation steps, and adapting the whole process for automation. This article explores microextraction techniques, selective approaches, on-line sample preparation, and dried matrix spots that aim to provide solutions to sample preparation problems in bioanalysis.
Photo Credit: simon2579/Getty Images

Lucie Nováková, Department of Analytical Chemistry, Faculty of Pharmacy, Charles University, Hradec Králové, Czech Republic
Sample preparation techniques in bioanalysis are multistep, time-consuming, and labour-intensive procedures that can take up 60–80% of the total analysis time. Sample preparation is often the limiting step of fast bioanalysis and the most error-prone part of the analytical method. There is currently a focus on improving the sample preparation process by shortening sample preparation time, cutting the cost of analysis, decreasing sample volume and solvent consumption, reducing the number of sample preparation steps, and adapting the whole process for automation. This article explores microextraction techniques, selective approaches, on-line sample preparation, and dried matrix spots that aim to provide solutions to sample preparation problems in bioanalysis.
The determination of drug concentrations in biological matrices is an important aspect of the drug development process. Bioanalytical methods are needed to obtain information on drug profiles from animal toxicokinetic studies and from clinical trials, including bioequivalence studies. These results are used to make critical decisions supporting the safety and efficacy of a drug substance or drug product. Biological fluids are not convenient for direct analysis using instrumental methods because of high sample complexity and the high content of many interfering compounds. Generally, very complex liquid samples including whole blood, plasma, serum, urine, or saliva are handled in bioanalysis. The target analytes are often present at very low concentrations, while the interfering compounds are abundant. Among the most important, salts and phospholipids are responsible for matrix effects in liquid chromatography–mass spectrometry (LC–MS) analysis. Proteins are damaging for the analytical instrumentation because they can irreversibly adsorb onto the stationary phase, resulting in a substantial loss of column efficiency, an increase in back pressure, or system clogging. To prevent these issues, adequate sample preparation is mandatory in bioanalytical methods (1,2,3).
With a well-designed sample preparation technique, isolation, cleanup, and preconcentration of the analytes of interest from the complex biological matrices can be achieved, while the interfering compounds can be removed. Sample preparation is an integral part of a bioanalytical method influencing all further steps of the analysis, with a crucial impact on the accuracy and precision of the final results. Unfortunately, it is still the most labour-intensive and timeâconsuming step of the analytical procedure, representing 60–80% of the total analysis time. This is in strong contrast to modern, fast LC methods and detection approaches and makes sample preparation a limiting step to fast bioanalysis (1,4).
The selection of an appropriate sample preparation technique is made with regards to the analyte type, sample type and amount available, requested selectivity and sensitivity of the procedure, extraction time, solvent consumption, and the possibility of automation. A well-designed sample preparation technique should involve a small amount of sample and a simple method, which is “just adequate” prior to analysis. This feature is important because complicated multistep procedures can introduce errors. Sample preparation techniques used for the treatment of biological fluids can be classified into two main groups based on the history and frequency of their use: conventional methods and modern approaches to sample preparation.
Conventional Sample Preparation Techniques
Conventional sample preparation approaches involve well-established and well-optimized techniques, which are commercially available and widespread across analytical laboratories. Straightforward, reliable, and high-throughput sample preparation techniques are a guarantee for achieving accurate results and meeting rigorous requirements for method validation in bioanalysis (5,6) within a reasonable time frame. These conventional approaches involve protein precipitation (PP), liquid–liquid extraction (LLE), and solid-phase extraction (SPE) (1,7,8).
Protein Precipitation (PP): Protein precipitation (PP) followed by centrifugation (or another precipitate separation step) belongs among the leading sample preparation techniques in modern bioanalysis, despite its very low selectivity and clean-up efficiency. Important benefits, such as very fast sample treatment, easy and fast method optimization, minimum number of steps, and no requirement for special equipment has brought this method to the attention of bioanalytical scientists. However, method sensitivity might be compromised and matrix effects may be serious. Another important drawback of PP is the difficulty of automating the centrifugation step. To facilitate the separation of precipitated phase and supernatant, new solutions have recently become available in the form of well-plates to enable filtration of the precipitated samples (1,7,8).
Liquid–Liquid Extraction (LLE): Liquid–liquid extraction is based on a transfer of the analyte from the aqueous sample to a waterâimmiscible solvent. Because of the high consumption of organic solvents and thus the production of a large volume of environmental pollutants in a conventional LLE setup, miniaturized versions of LLE are currently preferred. Such extraction is performed in glass vials or small test tubes (9) using typically 50–100 μL of sample and about 600–2000 μL of organic solvent (10,11). The amount of solvents and sample can be further decreased in modern microextraction approaches (see “Microextraction Techniques Based on LLE”).
LLE is a simple and straightforward extraction technique. Advantages include no requirement for special equipment or skills, lower cost compared to SPE, and potential to remove matrix effects in LC–MS because ionized compounds such as salts do not partition into the organic layer (3). Some disadvantages arise when largeâscale LLE is performed, such as bubble and emulsion formation. The high consumption of toxic organic solvents and production of a large amount of organic waste make LLE expensive and environmentally harmful. Another principal drawback of LLE is its unsuitability for hydrophilic compounds, which might be a significant issue in bioanalysis because some drugs and many metabolites possess polar structures. Last, but not least, most of the LLE procedures require the evaporation of nonpolar organic solvent and its reconstitution in the mobile phase to reach sample preconcentration and to obtain a sample compatible for injection into the LC system. This step substantially prolongs the extraction time and may be critical for sample recovery because of solubility issues (1,7,8,9). In order to improve the extraction efficiency, salting-out (SALLE) (12), sugaring-out (SULLE) (13), or in-vial derivatization approaches may be applied in LLE (9).
Although LLE is widely and commonly used in bioanalysis, its on-line coupling and full automation is difficult and requires substantial effort. An important approach to facilitate and automate LLE is supported liquid extraction (SLE). SLE columns contain specially processed wide-pore diatomaceous earth, a chemically inert matrix, which only acts as a holder for the aqueous sample. A 48- or 96-well plate format enables automation and high sample throughput using a positive-pressure manifold. The adoption of this approach has initially been very slow because of the well-established conventional LLE procedures in routine practice. However, there has been an increased interest in this arrangement as a result of this miniaturization and automation (14,15).
Solid-Phase Extraction (SPE): SPE is probably the most widely accepted sample preparation technique, enabling sample cleanup and preconcentration of the target analytes. The extraction of these analytes is based on partitioning between a solid phase (SPE cartridge) and a liquid phase (sample). The selection of SPE sorbent is the key factor, defining important parameters of selectivity, affinity, and capacity. SPE protocols are more selective compared to those of LLE because of the selectivity of these sorbents. Moreover, cleanup is also enabled during individual washing steps. Further advantages include lower consumption of organic solvents (compared to conventional LLE), high recovery, removal of nonvolatile salts, ease of operation, and ease of automation (1,16,17).
Currently, SPE is most widely performed in a conventional way using SPE cartridges. Other formats involve SPE discs and well plates. An SPE extraction protocol involves several steps, including sorbent activation and conditioning, sample load, a washing step, and, finally, elution of the analytes. It is performed using an SPE vacuum manifold, and so the requirements on equipment are slightly higher than in LLE or PP. It is important to point out that manual SPE is quite a time-consuming, multistep procedure, especially when the evaporation of SPE eluate and its reconstitution in mobile phase are needed. SPE is also relatively expensive because the cartridges are manufactured for single use only and require relatively large amounts of organic solvents. Therefore, modern development in SPE is focused on miniaturization (see “Microextraction Techniques Based on SPE”), automation (see “On-Line Sample Preparation Techniques”), enhanced selectivity (see “Sample Preparation with High Selectivity”), and new materials (see “New Materials in Sample Preparation”) (1,7).
Because phospholipids are an important source of matrix effects, the use of hybrid precipitation and SPE plates for the simultaneous removal of precipitated proteins and phospholipids has recently become popular (18).
Modern Advances in Sample Preparation
The research and development of modern sample preparation techniques is focused on miniaturization and facilitation of the sample preparation step. One of the main goals is to decrease both sample and solvent volumes, to be in agreement with the green principles in chemistry (19). Further requirements include simpler equipment, a reduction of handling steps, and a shortening of sample preparation time, with the final objective of reducing sample preparation cost and the susceptibility to errors (1,7,8). As ultrahigh-performance LC (UHPLC) and other fast-LC approaches are now replacing conventional high performance LC (HPLC) methods, there is a need for fast and high throughput sample preparation techniques.
Microextraction Techniques Based on LLE: Microextraction approaches based on liquid-phase extraction are a very extensive group of techniques. They may use very simple devices, quite a complex setup, or membrane assistance to achieve better phase separation (20). These techniques provide important benefits such as almost solventless extraction and very high preconcentration factors (21). Basic classification of the liquidâphase microextraction techniques based on the extraction principle involves single drop microextraction (SDME), dispersive liquid–liquid microextraction (DLLME), and membrane-supported–LLE. The membrane techniques include various hollow-fibre liquidâphase microextraction (HF-LPME) configurations (22) and its advanced variant electromembrane extraction (EME) (21). The last development of membrane supported-LLE is represented by parallel artificial liquid membrane extraction (PALME). Despite a growing number of applications of liquid-phase microextraction approaches in the scientific literature, the adoption in routine bioanalytical laboratories is much slower. Indeed, their use may be limited in routine bioanalysis because of the following drawbacks: manipulation with the devices may require skilful operation; method development may be time-consuming in more complex setups; longer extraction times compared to other techniques to accomplish the same task; and only a small preconcentration factor obtained from a low amount of biological sample compared to other matrices, where the enrichment factors can be much higher. Among the discussed LLEâbased microextraction approaches, PALME (23,24), EME (25) - both in 96-well plate format - and DLLME show the greatest potential in bioanalysis. The latter has been more widely applied in many fields and also in bioanalysis, particularly because of its speed and ease of use, which is unique among the LLE-based microextraction approaches (20).
To perform DLLME, an immiscible solvent and a miscible disperser solvent are injected into an aqueous sample, leading to the formation of a cloudy solution of fine droplets of an extraction solvent. As a result of the high surface contact between extraction solvent droplets and aqueous samples, high recovery and enrichment factors are obtained even when using very small volumes of the extraction solvent (20).
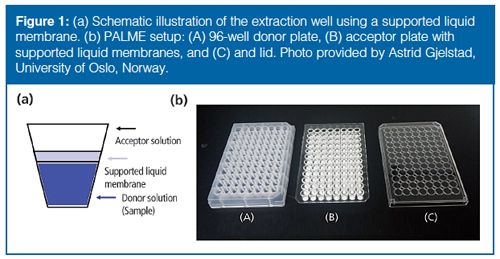
PALME has been recently developed to take advantage of clean extracts obtained when using back-extraction and to facilitate the automation of the LLE procedure (23,24). Similar to HF-LPME, the analytes are extracted with the help of a supported liquid membrane (SLM). The extraction setup is composed of a 96-well donor and acceptor plates (Figure 1). The acceptor plate contains 96 artificial membranes that are impregnated with an organic solvent to create the SLMs. The pH gradient across the SLM serves as the driving force for mass transfer. The aqueous acceptor solution can be directly analyzed with LC–MS/MS (23).
EME is another technique using a similar setup to the one used in HF-LPME (SLM) with the addition of a power supply and two electrodes. A power supply provides d.c. potential to enhance the extraction rate of ionizable analytes from the donor solution (sample) to the acceptor phase, which is contained in the lumen of a hollow fibre (25).
Microextraction Techniques Based on SPE: Microextraction approaches based on SPE involve three different groups of extraction techniques using diverse extraction principles. A miniaturized version of SPE is applied in microextraction by packed syringe (MEPS) and µ-SPE in pipette tips (µ-SPE-PT). In MEPS, the extraction is made using a specially adapted syringe handled manually or with an automated analytical syringe (26,27). The solid packing material (1–4 mg) is inserted into the barrel of a syringe as a plug or between the needle and the barrel as a cartridge. In µ-SPEâPT, the sorbent is firmly placed into the pipette tip. Both commercially available or laboratory-made sorbents can be used for extraction. A great advantage of the latter is the option to tailor-make sorbents using a combination of different chemistries (Figure 2). This approach originates from proteomics and is sometimes designated as STop And Go Extraction tips (StageTips) (28,29,30). In µ-SPE-PT extraction procedures, pipetting or centrifugation may be used as a driving force to perform the individual steps of SPE. The latter enables simultaneous treatment of a large number of samples (Figure 2[c]).
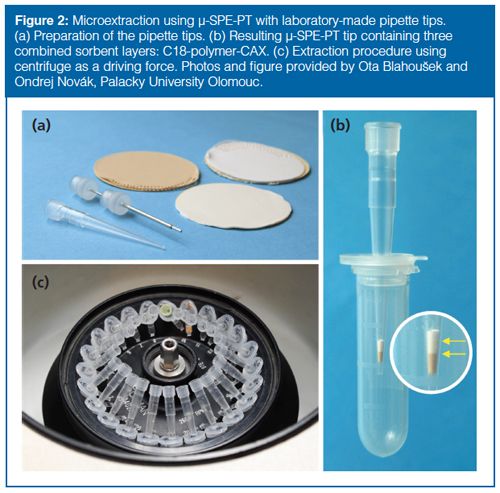
In both µ-SPE-PT and MEPS techniques, the same protocol used for conventional SPE is applied with much lower sample and solvent volumes. Straightforward method development (a result of the similarity with conventional SPE) is another very important benefit. It is easy and straightforward to automate the procedure in µ-SPE-PT, but somewhat more challenging for MEPS, requiring a dedicated autosampler.
The second approach, which is called dispersive SPE (d-SPE), is used in µ-dSPE-PT or in QuEChERS (quick, easy, cheap, effective, rugged, and safe) extractions. Compared to the previous approach, it differs in the sorbent placement and the extraction process. In µ-dSPE-PT, the extraction also takes place in the standard pipette tips. The sorbent is loosely placed between the two frits. This variant is also designated as disposable pipette tips extraction (DPX). However, sorbent price is higher and stationary phase choice is more limited compared to traditional SPE (31). Individual extraction steps are performed using a standard pipette, which allows the sorbent to be dynamically mixed with the extracted material and leads to a rapid adsorption equilibrium between solid phase and the analyte. In this way, every sorbent particle actually faces analyte several times. This is contrary to conventional SPE, where a greater amount of sorbent is needed because the analyte comes into contact with the sorbent particles only once. This extraction procedure leads to fast and efficient extractions (8).
The third solid-phase–based extraction principle is different from conventional SPE. Here, adsorption, absorption, and desorption processes take place on the solid sorbent, allowing the need for solvents to be completely eliminated. The sorbent is placed on the fused-silica fibre in the modified syringe in solid-phase microextraction (SPME) (32,33), in a piece of capillary in in-tube SPME (32), or on the stir bar in stirâbar sorptive extraction (SBSE) (34). In SPME, the coated fibre can be moved in and out using a plunger. Using such simple equipment, all steps - extraction, preconcentration, derivatization, and transfer to the chromatographic system - are integrated into one device. Various materials such as PDMS (polydimethylsiloxane) or many other materials may be used for the fibre coating. New types of SPME fibres use biocompatible coatings prepared by immobilizing various sorbents with polyacrylonitrile (35). Since 1990, when SPME was first introduced, a series of modifications and improvements have been proposed. Initially, either direct immersion (DI-SPME) or head-space fibre (HS-SPME) were applied; however, in vivo SPME sampling devices and fully automated 96-well format multifibre SPME are now preferred (33). The main benefits of SPME include no need for solvents, the ease of automation, minimal equipment requirements, good linearity, and relatively high sensitivity. Some of the drawbacks include a longer time needed for extraction, limited capacity and fragility of SPME fibre, generally lower recoveries than those using LLE and SPE, and the incidence of carryover effects. The use of modern SPME in routine laboratories is still limited.
On-Line Sample Preparation Techniques: The use of on-line sample preparation techniques reduces sample manipulation and provides high preconcentration factors, recoveries, high speed, and throughput. SPE using 96- or 384-well plate formats in fully automated robotic workstations, semi-automated, or in an off-line configuration is therefore the most well-established technique in bioanalysis (36,37).
On-line SPE technology in combination with LC–MS/MS analysis has experienced many developments in recent years and enables faster and precise determination in both conventional and miniaturized arrangements (see “Microextraction Techniques Based on SPE”). On-line hyphenation is accomplished using switching valves and an additional pump. The experimental parameters to be optimized in on-line SPE are the type of sorbent, the solvents used in the different SPE steps, and their flow-rates (extraction time). In addition, further pretreatment, such as filtration, centrifugation, or protein precipitation, might sometimes be required prior to on-line SPE. Other constraints may include reduced sorbent capacity, too strong retention, slow kinetic of the sorption process, and possible absorption of the analyte on the system tubes (9,36,38). Specific approaches to remove macromolecules from the biological fluids include molecularly imprinted polymers (MIPs), restrictedâaccess media (RAM), and turbulent flow chromatography (TFC).
RAM sorbents are used for the direct injection of biological fluids into a chromatographic system to enable the fractionation of a biological sample into a protein matrix and analyte fraction. This will lead to the extraction and enrichment of low molecular compounds into the interior phase via partition. The exclusion of macromolecules can be accomplished using the outer surface of the RAM particle as a physical or a chemical barrier. The use of RAM sorbents for the direct and repeated analyses of biological fluids is already well established. However, the complete elution of the analytes from RAM on the analytical column might sometimes be quite challenging (36,39).
TFC enables the direct injection of biological fluids and the separation of small analyte molecules from the macromolecular matrix based on the low-diffusion coefficients of proteins using turbulent flow. The generation of turbulentâflow requires short, narrow-bore columns, packed with large-size particles (typically 50 mm × 1.0 mm, 20–60 μm) and flow-rates in the range of 4–5 mL/min. Highâmolecular-weight compounds are quickly eluted using a pure water or buffer mobile phase and are usually directed to the waste. The retained small molecular weight analytes are subsequently eluted onto an analytical column for the chromatographic separation using an organic mobile phase (36).
To increase the limited capacity and facilitate its automation, on-line coupling of SPME with separation techniques has been established and termed in-tube SPME. In this setup, a capillary column is placed as the injection loop in a standard autosampler. While the typical advantages attributed to the on-line setup are obtained with this technique, there are also several drawbacks, such as the need for more complex instrumentation with commercially unavailable adjustments; a requirement for very clean samples, because the capillary can be easily clogged; low extraction efficiency; selectivity; and mechanical stability. To overcome these issues, development is focused on coupling in-tube SPME with miniaturized LC techniques and the preparation of new extraction phases (36,40,41).
Sample Preparation with High Selectivity: The selectivity of SPE can be further improved using specific sorbents, such as MIPs, immunoaffinity sorbents, or aptamers.
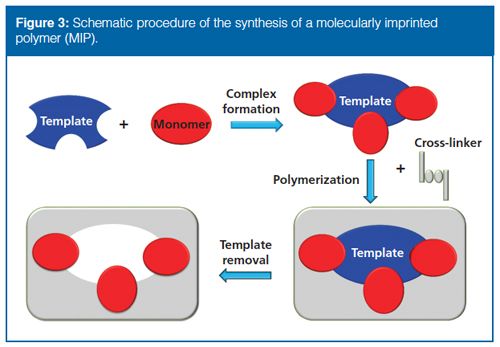
MIPs are stable synthetic polymers possessing tailorâmade recognition sites, which can specifically retain target analyte molecules. The preparation of MIPs involves the copolymerization of a complex formed by the template molecule and a functional monomer with a cross-linking agent in the presence of a suitable porogenic solvent (Figure 3). After removing the template, the resulting cavity is complementary to the target analytes in terms of size, shape, and functionality. An SPE cartridge is the most popular form of MIP used in sample preparation. However, growing interest in miniaturization has led to the use of MIPs in SPME, SBSE, MEPS, µ-SPE, membranes, and magnetic beads. High extraction selectivity and capability to eliminate the matrix effects are definitely the most important benefits of MIPs. Other advantages include reusability, ease of use, and low cost of preparation. Some features that need to be improved in future research include the need for higher yields of specific binding sites, the need for some rules for MIP design, and the application of MIPs to aqueous samples (42,43).
Immunoaffinity-based SPE (IA-SPE) uses sorbents with attached antibodies to obtain high selectivity from antigen–antibody interaction. For small molecules, the target analyte is typically coupled to the carrier protein (hapten) to achieve an immune response. While the crossâreactivity of antibodies with structural analogues is considered as a negative feature in immunoassay, it is exploited in immunoaffinity extraction. An important limitation of IA-SPE is the difficult, expensive, and timeâconsuming synthesis of antibodies with no certainty of success, which probably prevents wider use of IA-SPE in routine bioanalytical laboratories (44).
Aptamer-functionalized materials (AFMs) are promising, specific-recognition materials in sample preparation, providing many advantages such as high specificity and binding affinity, good stability, low cost, nontoxicity, ease of synthesis, and easy and controllable modification. Aptamers are the artificial single-stranded oligonucleotides generated by an in vitro selection process called SELEX (systematic evolution of ligands by exponential enrichment). By folding into distinct secondary or tertiary structures, aptamers can bind to certain targets with extremely high specificity. So far, they have been widely used in biosensors or in immunoassays, while their use in sample preparation in bioanalysis is still at the research stage (45,46).
New Materials in Sample Preparation: New types of materials have been introduced in line with the green analytical chemistry concept. Among them are sol-gel-based materials, which allow inorganic and organic-inorganic hybrid polymers with typically higher thermal and chemical stability, controlled morphology, surface properties, and pore structures to be obtained due to the controlled synthesis conditions. These sorbents are successfully used in SPE, SBSE, SPME, and MEPS (47).
Ionic liquids (ILs) are used to enhance extraction efficiency and selectivity in LLE-based procedures and as sorbents in SPE-based techniques. They are liquid salts at room temperature, with a melting point lower than 100 ºC. Their structure consists of organic cations derived from Lewis bases and polyatomic anions. The addition of different structures enables their hydrophobic or hydrophilic abilities to be tailored (47).
Along with the development of nanotechnology, various nanomaterials have been introduced into the sample preparation domain. Nanomaterials refer to a special kind of materials with nanometric scales. Compared to conventional materials, some exceptional properties, such as ultrahigh specific area and increased surface activity, are facilitating the application of nanomaterials in sample preparation. Further advantages include tunable compositions, various morphologies, and flexible functionalization. Nanomaterials used in sample preparation include nanoparticles and nanoporous materials (48,49).
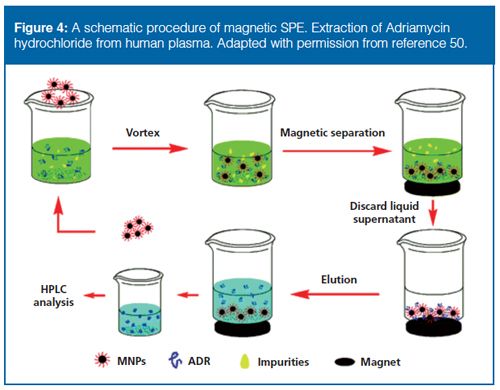
Magnetic separation techniques (Figure 4) (50) are an interesting approach used in sample preparation and provide several advantages, such as efficient, gentle, and nondestructive separation, especially for large molecules. Magnetic separation techniques are therefore able to facilitate or accelerate many separation and purification procedures because of rapid isolation of analytes using an external magnetic field (51,52).
Dried Matrix Spot Analysis: Dried matrix spot (DMS) analysis has recently gained attention in bioanalytical routine laboratories. Although dried blood spot (DBS) analysis is the most widely used (53,54), other matrices, such as dried saliva spots (DSS) (55), dried plasma spots (DPS) (56), dried urine spots (DUS), and even cerebrospinal fluid (CSF) sample spots (57), have been successfully analyzed.
The DBS and other matrix spot sampling is minimally invasive and uses substantially lower sample volumes (<100 µL for four spots) compared to other procedures. DBS uses capillary blood from a finger prick with a lancet. After collection, the samples are dried on the special sample collection cards. As the samples are collected, transported, and stored in dry form, the handling and storage of such biological material becomes easier and may lead to increased stability of some unstable compounds. For subsequent analysis, the disk is punched out from the spot and this disk is extracted for the isolation of target analytes, which is typically followed by LC–MS/MS. Despite some challenges, such as proper DBS calibration, analyte stability, the influence of hematocrit on blood spot size, and the need for clinical validation because of the differences between venous, whole blood, and plasma, its wider use in bioanalysis is expected in the future, as it is a fast, relatively simple, and straightforward sample preparation technique (53,54). The importance of microsampling in bioanalysis is confirmed by the development of a new microsampling approach termed volumetric absorptive microsampling (VAMS). This approach should compensate for the area bias and homogeneity issues associated with conventional DBS (58,59).
Conclusions
Modern method development in bioanalysis is focused on speed of analysis, efficiency, selectivity, sensitivity, low cost, miniaturization, and automation to obtain high sample throughput and high quality data. While significant improvements have been made in instrumental methods - both in chromatographic separation and MS/MS detection - the sample preparation step still remains the most time-consuming and labour-intensive aspect of a bioanalytical method, particularly when compared to ultraâfast chromatography. The efforts to improve this situation have resulted in the development of many new sample preparation techniques, which have helped to overcome some of the drawbacks of the conventional sample preparation techniques.
References
- L. Nováková, J. Chromatogr. A1292, 25 (2013).
- V. Pucci, S. Di Palma, A. Alfieri, F. Bonelli, and E. Monteagudo, J. Pharm. Biomed. Anal.50, 867 (2009).
- M. Jemal, Z. Ouyang, and Y.-Q. Xia, Biomed. Chromatogr. 24, 2 (2010).
- A. Kaufmann, Trends Anal. Chem.63, 113 (2014).
- Committee for Medicinal Products for Human Use, Guideline on Bioanalytical Method Validation (EMA, London, UK, 2011).
- U.S Department of Health and Human Services, Food and Drug Administration, Guidance for Industry, Bioanalytical Method Validation (2001).
- L. Nováková and H. Vlcková, Anal. Chim. Acta656, 8 (2009).
- P. L. Kole, G. Venkatesh, J. Kotecha, and R. Sheshala, Biomed. Chromatogr. 25, 199 (2011).
- L. Ramos, J. Chromatogr. A 1221, 84 (2012).
- J.-W. Gao, Y.-M. Yuan, Y.-S. Lu, and M.-C. Yao, Biomed. Chromatogr.26, 1482 (2012).
- A.E.-H. de Mendoza, I. Imbuluzqueta, M.A. Campanero, D. Gonzalez, A. VilasâZornoza, X. Agirre, H. Lana, G. Abizanda, F. Prosper, and M.J. BlancoâPrieto, J. Chromatogr. B879, 3490 (2011).
- D. Jain, R. Athawale, A. Bajaj, and S. Shrikhande, J. Chromatogr. B 970, 86 (2014).
- J. Zhang, F. Myasein, H. Wu, and T.A. El-Shourbagy, Microchem. J.108, 198 (2013).
- S.X. Peng, T.M. Branch, and S.L. King, Anal. Chem.73, 708 (2001).
- L. Nováková, V. Desfontaine, F. Ponzetto, R. Nicoli, M. Saugy, J.-L. Veuthey, and D. Guillarme, Anal. Chim. Acta915, 102 (2016).
- M.C. Henion, J. Chromatogr. A856, 3 (1993).
- A. Andrade-Eiroa, M. Canle, V. LeroyâCancellieri, and V. Cerdà, Trends Anal. Chem.80, 641 (2016).
- C. Bylda, R. Thiele, U. Kobold, and D.A. Volmer, Analyst139, 2265 (2014).
- J. PÅotka, M. Tobiszewski, A.M. Sulej, M. Kupska, T. Górecki, and J. Namiesnik, J. Chromatogr. A1307, 1 (2013).
- J.A. Ocaña-Gonzáles, R. FernándesâTorres, M.Á. Bello-Lopéz, and M. Ramos-Payán, Anal. Chim. Acta905, 8 (2016).
- F. Pena-Pereira, I. Lavilla, and C. Bendicho, Spectrochim. Acta Part B64, 1 (2009).
- S. Pedersen-Bjergaard and K.E. Rasmussen, J. Chromatogr. B817, 3 (2005).
- K.S. Ask, T. Bardakci, M.P. Parmer, T.G. Halvorsen, E.L. Øiestad, S. PedersenâBjergaard, and A. Gjelstad, J. Pharm. Biomed. Anal.129, 229 (2016).
- L.E.E. Eibak, M.P. Parmer, K.E. Rasmussen, S. Pedersen-Bjergaard, and A. Gjelstad, Anal. Bioanal. Chem.406, 431 (2014).
- L.E.E. Eibak, K.E. Rasmussen, E.L. Øiestad, S. Pedersen-Bjergaard, and A. Gjelstad, Anal. Chim. Acta828, 46 (2014).
- M. Abdel-Rehim, Anal. Chim. Acta701, 119 (2011).
- M.M. Moein, A. Abdel-Rehim, and M. Abdel-Rehim, Trends Anal. Chem.67, 43 (2015).
- J. Rappsilber, Y. Ishihama, and M. Mann, Anal. Chem.75, 663 (2003).
- Y. Ishihama, J. Rappsilber, and M. Mann, J. Proteome Res. 5, 988 (2006).
- J. Svacinová, O. Novák, L. Placková, R. Lenobel, J. Holík, M. Strnad, and K. Doležal, Plant Methods8, 17 (2012).
- D.C.M. Bordin, M.N.R. Alves, E.G. de Campos, and B.S. De Martinis, J. Sep. Sci. 39, 1168 (2016).
- H. Kataoka and K. Saito, J. Pharm. Biomed. Anal.54, 926 (2011).
- B. Bojko, E. Cudjoe, G.A. Gómez-Ríos, K. Gorynski, Ruifen Jiang, N. Reyes-Garcés, S. Risticevic, É.A.S. Silva, O. Togunde, D. Vuckovic, and J. Pawliszyn, Anal. Chim. Acta750, 132 (2012).
- F.J. Camino-Sánchez, R. RodríguezâGómez, A. Zafra-Gómez, A. Santos-Fandilla, and J.L. Vílchez, Talanta 130, 388 (2014).
- F.S. Mirnaghi, Y. Chen, L.M. Sidisky, and J. Pawliszyn, Anal. Chem.83, 6018 (2011).
- O. Núñez, H. Gallart-Ayala, C.P.B. Martins, P. Lucci, and R. Busquets, J. Chromatogr. B927, 3 (2013).
- L. Krcmova, D. Solichova, and P. Solich, Talanta115, 973 (2013).
- M. Rogeberg, H. Malerod, H. RogergâLarsen, C. Aass, and S.R. Wilson, J. Pharm. Biomed. Anal.87, 120 (2014).
- S. Souverain, S. Rudaz, and J.-L. Veuthey, J. Chromatogr. B801, 141 (2004).
- M. Fernández-Amado, M.C. PrietoâBlanco, P. López-Mahía, S. Muniategui-Lorenzo, and D. Prada-Rodríguez, Anal. Chim. Acta906, 41 (2016).
- Y. Moliner-Martinez, R. HerráezâHernández, J. Verdú-Andrés, and C. Molins-Legua, Trends Anal. Chem. 71, 205 (2015).
- Y. Hu, J. Pan, K. Zhang, H. Lian, and G. Li, Trends Anal. Chem.43, 37 (2013).
- E. Turiel and A. Martín-Esteban, Anal. Chim. Acta 668, 87 (2010).
- N. Delaunay, V. Pichon, and M.-C. Hennion, J. Chromatogr. B15 (2000).
- Q. Yuan, D. Lu, X. Zhang, Z. Chen, and W. Tan, Trends Anal. Chem.39, 72 (2012).
- F. Du, L. Guo, Q. Qin, X. Zheng, G. Ruan, J. Li, and G. Li, Trends Anal. Chem. 67, 134 (2015).
- B.H. Fumes, M.R. Silva, F.N. Andrade, C.E. Domingues, and F.M. Lanças, Trends Anal. Chem. 71, 9 (2015).
- L. Xu, X. Qi, X. Li. Y. Bai, and H. Liu, Talanta 146, 714 (2016).
- Y. Wen, L. Chen, J. Li, D. Liu, and L. Chen, Trends Anal. Chem.59, 26 (2014).
- N. Ma, L. Zhang, R. Li, Y. Zhou, Z. Cai, Ch. Dong, and S. Shuang, Anal. Methods6(17), 6736–6744 (2014).
- J. He, M. Huang, D. Wang, Z. Zhang, and G. Li, J. Pharm. Biomed. Anal. 101, 84 (2014).
- M. Wierucka and M. Biziuk, Trends Anal. Chem. 59, 50 (2014).
- Y. Enderle, K. Foerster, and J. Burhenne, J. Pharm. Biomed. Anal. in press (2016).
- N. Zheng, L. Yuan, Q.C. Ji, H. Magnus, Y. Song, Ch. Frost, J. Zeng, A.-F. Aubry, and M.E. Arnold, J. Chromatogr. B 988, 66 (2015).
- N. Zheng, J. Zheng, Q.C. Jin, A. Angeles, A.-F. Aubry, S. Basdeo, A. Buzescu, I.S. Landry, N. Jariwala, W. Turley, R. Burrell, and M.E. Arnold, Anal. Chim. Acta in press (2016).
- W. Li, J. Doherty, S. Favara, Ch. Breen, J. Flarakos, and F.L.S. Tse, J. Chromatogr. B991, 46 (2015).
- B. Rago, J. Liu, B. Tan, and Ch. Holliman, J. Pharm. Biomed. Anal.55, 1201 (2011).
- P. Dennif and N. Spooner, Anal. Chem.86(16), 8489–8495 (2014).
- L. Mercolini, M. Protti, M.C. Catapano, J. Rudge, and A.E. Sberna, J. Pharm. Biomed. Anal.123, 186 (2016).
Lucie Nováková is an associate professor in the Department of Analytical Chemistry, Charles University, Faculty of Pharmacy in Hradec Králové, Czech Republic. She has a Masters degree in pharmacy and obtained her Ph.D. in pharmaceutical analysis from Charles University, Faculty of Pharmacy. Her research is focused on fast LC and SFC techniques, especially UHPLC, UHPSFC, and their coupling to MS. She is involved in a wide variety of research projects focused on pharmaceutical analysis, plant analysis, doping control, and bioanalytical methods. An important part of her research therefore lies in the sample preparation step, where the focus is put on the current trends enabling facilitation, miniaturization, and reduction of time and sample requirements. She has published over 75 scientific articles with about 1650 citations and is widely involved in teaching and education activities, such as HPLC and SFC training courses, seminars, and congresses.


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








