Accurate Mass Screening for Pesticide Residue Testing
The Application Notebook
Bruker Daltonics
James Hillis, Petra Decker, Ian Sanders and Carsten Baessmann, Bruker Daltonics
The update to the SANCO guidelines in 2009 recognized the power and potential of time-of-flight (TOF) mass spectrometry for pesticide screening applications. When compared with historical screening tools (such as the use of triple-quad mass spectrometry), high performance accurate mass TOF systems offer several advantages for screening applications. These advantages include better resolution, unlimited multiple target capabilities and the possibility to perform comprehensive and/or retrospective analysis on samples in silico at will.
The maXis impact™ continues the evolution of Bruker's unique TOF accurate mass instruments. The performance of ≥40k resolution, ≤1ppm mass accuracy and high isotopic fidelity is maintained without sensitivity compromise. While acquisition at up to 50 Hz enables maXis impact to handle the fastest of ultra high performance liquid chromatography (UHPLC) separations with ease. Additionally, to aid in quantitative applications, the maXis impact delivers an increase in sensitivity of up to 50-fold over previous generation instruments, especially at the low m/z tandem mass spectrometry (MS–MS) signals important for analyte confirmation.
The dramatic increase in sensitivity changes the game for pesticide screening applications. With limits of detection (LODs) typically in the sub ng/mL levels, achieving excellent maximum residue levels (MRLs) becomes very easy. The maXis impact enables analysts to obtain both qualitative and quantitative results in a single analysis. Identification data including accurate mass MS and MS–MS, isotopic pattern fitting and Rt fitting can be readily collected and correlated in dedicated software. In the same run, sub ng/mL limits of quantification (LOQs) with dynamic ranges over four orders of magnitude can also be obtained (Figure 1).

Figure 1: Fluoxastrobin at 50 fg to 1 ng on column.
Taken together, the key qualitative and quantitative performance attributes of the maXis impact make it an excellent choice for routine pesticide screening applications with confirmation and quantitation in a single run. The maXis impact can provide comprehensive data quality that is unmatched by any other technology currently available.
Experimental
A mixture of 200 pesticides was run on the PesticideScreener™ application. This mixture has previously been well characterized and publicized. This same mixture was used to assess the performance of the maXis impact and specifically to assess the increase in sensitivity observed.

Conclusions
The maXis impact changes the game in small molecule pesticide screening. Sensitivity increases of 20- to 50-fold versus previous systems are routine. This enhanced sensitivity, coupled with the additional capabilities of the maXis impact, allows both high quality qualitative and quantitative data to be acquired in a single run (Figure 1). The capabilities of the maXis impact are ideally suited to pesticide screening and quantification, and provides an unrivalled level of confidence at sub ppb levels.
To further optimize the use of the maXis impact for pesticide screening, the PesticideScreener application platform is a fully developed protocol, allowing the use of accurate mass and isotopic pattern fitting and retention time fitting, for straight-out-of-the-box implementation of this outstanding analytical system (Figure 2).
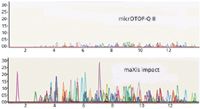
Figure 2: Overlapped hrEIC traces showing the evolution of TOF sensitivity.
The maXis impact sets the new gold standard for pesticide screening through full utilization of the capabilities of ultrahigh-sensitivity TOF accurate mass spectrometry.

Bruker Daltonics Inc.
40 Manning Road, Billerica, Massachusetts, USA
tel. (978) 663-3660, fax (978) 667-5993
Website: www.bruker.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.












