A Novel 3D-Printing Method to Create Liquid Chromatography Columns
For approximately a decade, three-dimensional (3D)-printed columns have been hailed as the future of liquid chromatography (LC). However, the resolution of typical printing methods has fallen short of the requirements to make an effective analytical- or preparative-scale column. In this article, we describe a new 3D-printing method that can create large-volume columns with a feature resolution of 10 µm.
During the ongoing quest for fundamental advancements in high performance liquid chromatography (HPLC) column performance in the past two decades, several methods of producing ordered stationary phases have been explored. To quote John Knox, “The overall effect of a very homogenous bed is to greatly enhance chromatographic efficiency” (1). Over the past decade, the emergence of additive manufacturing, or three-dimensional (3D)-printing, led to many in the chromatography field to consider it as the eventual method to create ordered stationary phases with tailored geometries (2).
Studies have been performed on orientations (3), element shapes (4), and novel porous geometries that are best suited for 3D-printed columns. The efficiency of such structures were demonstrated in both computational simulations and experimental tests, with reduced chromatographic plate heights as low as 1.12. Furthermore, a bifunctional resin has been developed that allows for direct printing of anion exchange columns (5,6).
However, a limitation in such studies has been the feature size of the porous beds that were created. This limitation is because of the limited resolution of most 3D-printing methods. Common printing methods, such as fused deposition modelling, stereolithography, and selective laser sintering, typically operate with nominal resolutions in the order of 20–100 µm. By operating with nominal resolutions, common printing methods generate feature sizes of porous beds typically greater than 300 µm, which is two orders of magnitude short of the feature sizes that are necessary for use in HPLC columns.
In contrast to conventional methods, the high-resolution 3D-printing method called two‑photon polymerization is capable of creating pore sizes of under 1 µm with high fidelity. However, as detailed in a previous article by De Malsche and coworkers (7), the printing method requires impractically long production times for analytical-scale columns typically used in HPLC. Therefore, the current capabilities of 3D-printing technologies are inadequate to create analytical- or preparative‑scale columns because of limitations in both resolution and print speeds.
To address these limitations, we have developed a 3D-printing technique called hybrid stereolithography (HSLA) (8), which combines traditional stereolithography and photolithography, and is capable of high-resolution and fast printing of high-volume structures.
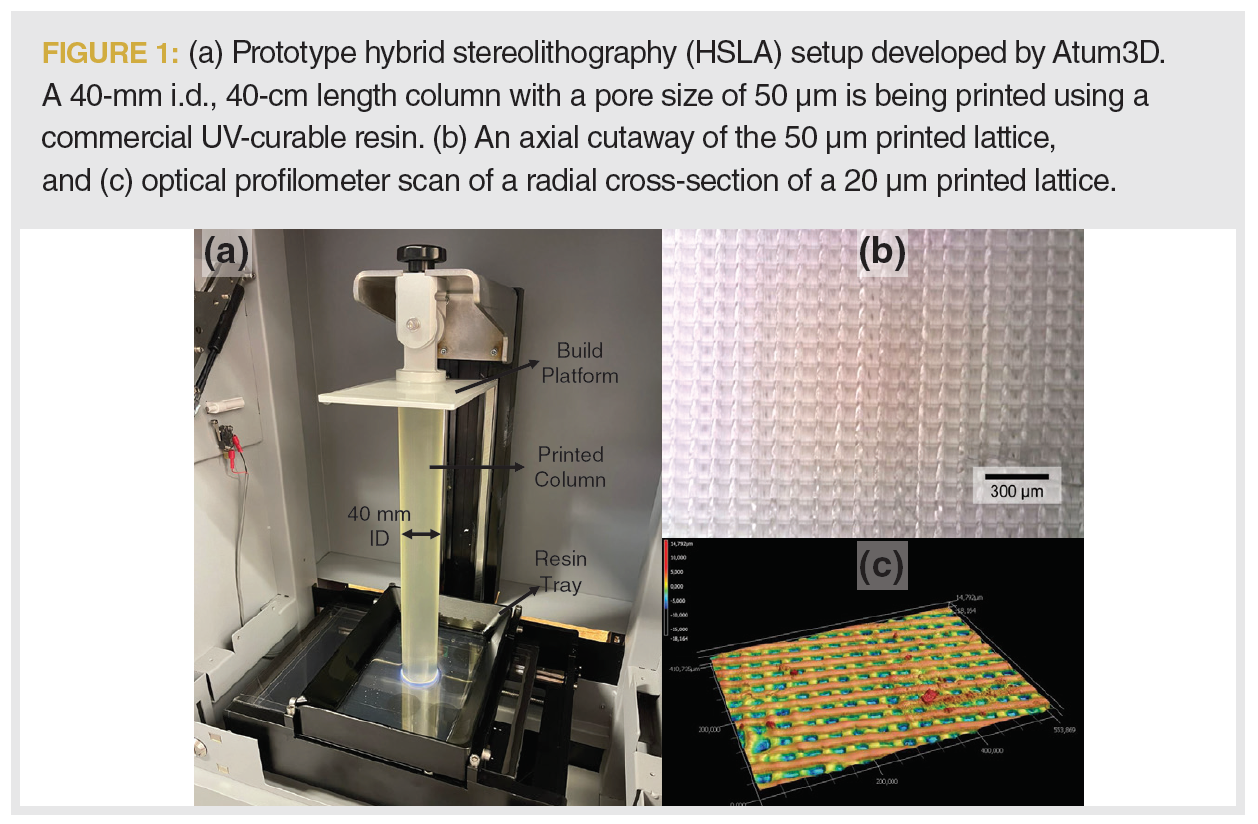
Figure 1(a) shows a prototype HSLA setup during the printing process. A lower resolution pattern is illuminated, using an ultraviolet (UV) light source (shown in blue in Figure 1[a]) and a digital mirror device. To achieve higher resolution than traditional stereolithographic methods, a high-resolution photomask with predefined patterns is used between the resin tray and the digital pattern. After a layer is cured, the build platform lifts to peel the printed piece from the resin tray.
The primary advantage of such a system is that it prints high‑resolution repetitive features such as ordered LC column microstructures. The photomask patterns can be used to print grids with feature sizes as low as 10 µm without compromising on printing speeds. Because all regions of a pattern are cured simultaneously, the column internal diameter (i.d.) is entirely independent of the print speed. For example, the 40-mm i.d. column seen in Figure 1(a) was printed at a speed of 5 mm of column length per hour, translating to 6.5 mL/hr. The 40-mm i.d. column represents an improvement of greater than three orders of magnitude compared to the fastest two photon polymerization systems for a similar resolution (9). The high printing speed can be used to create large preparative‑scale columns, or several analytical‑scale columns in parallel (up to 32 4.6-mm i.d. columns in the setup shown in Figure 1[a]).
Figures 1(b) and 1(c) show axial and radial cutaways of two different lattices printed using HSLA. Figure 1(b) shows a 50 µm simple cubic grid printed in the setup shown above, with a design porosity of 50% and a layer thickness of 25 µm. Figure 1(c) shows a lattice with a feature size of 20 µm with a layer thickness of 10 µm. In both cases, the ordered nature of the printed structures is evident, with the photomask features being imprinted on the structure with good fidelity.
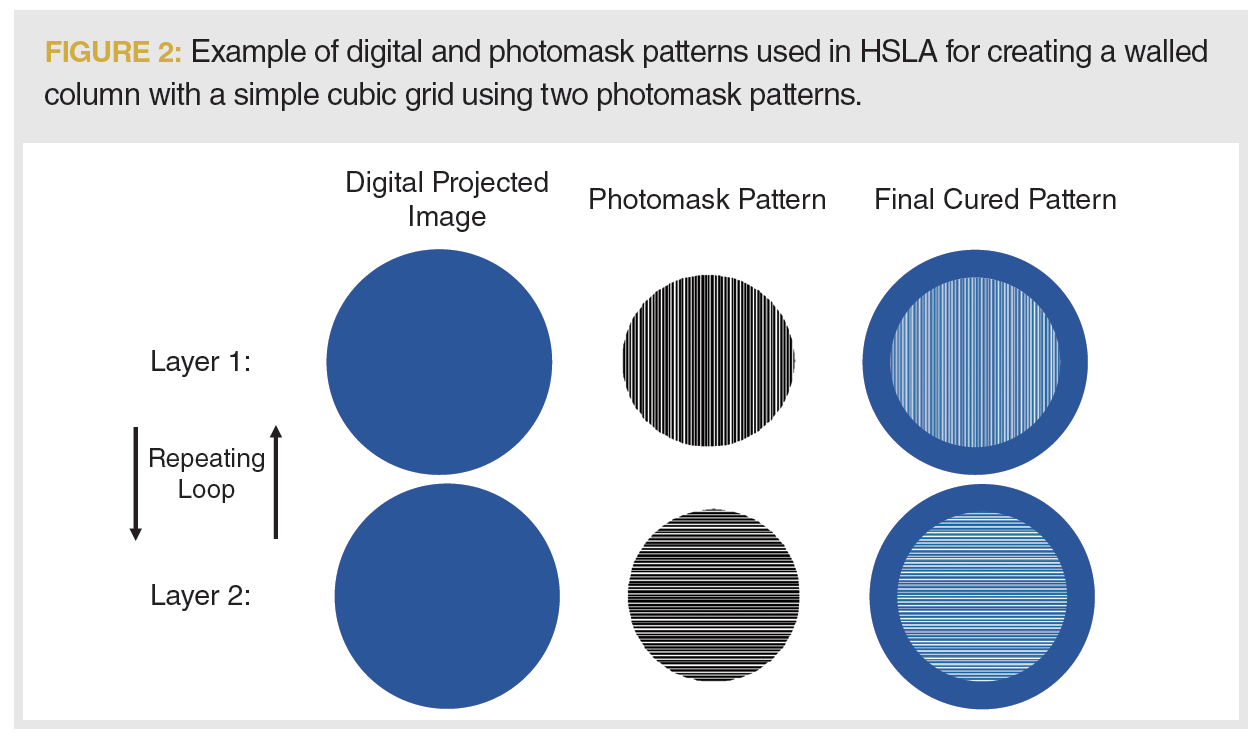
To create a 3D structure, several patterns were defined on the photomask, with a linear stage moving the photomask after a layer is cured. A photomask-switching system with two patterns, shown in Figure 2, was used to create the lattice seen in Figure 1(b). More complex 3D structures can also be created using more photomask patterns. Because LC column microstructures typically consist of simple, repeatable geometries, a photomask-switching system is well-suited for column production.
A commercial nonporous high‑resolution photopolymer resin called Nanoclear (FTD, Alkmaar) was used for all tests. For this study, 1-, 2.1-, 4.6-, 10-, and 20‑mm i.d. columns were produced and thoroughly flushed using isopropanol to clear the porous structure of uncured resin. An external casing with fittings and flow distributors was used to connect the column to an HPLC system.
To perform residence time distribution (RTD) tests on the printed 4.6-mm i.d. × 5-cm L structures, 1 µL injections of 1 mg/mL of uracil were performed on a Waters Acquity H-Class system across a range of flow rates (0.01–4 mL/min). For the RTD tests, two flow orientations were tested—with and against the print direction of the column, which is shown in Figure 3(a). In addition to the RTD tests, pycnometric measurements were performed to determine the total void volumes of the printed columns.
Uracil injections were performed in triplicate on the 4.6-mm i.d. columns from two directions. The resulting plate heights show a classical van Deemter curve, with a clear column efficiency minimum (Qmin) of 0.25 mL/min. As seen in Figure 3(b), aligning the flow direction with the print direction (that is, top to bottom as seen in Figure 3[a]) is clearly advantageous compared to orienting the column against the direction of the print. A minimum absolute plate height of H = 112.4 µm (a reduced plate height of h = 2.25) was demonstrated with the column oriented in the direction of the print. To the best of our knowledge, these represent the lowest absolute plate heights for 3D-printed columns, and a fivefold improvement compared to previous tests using conventional printing methods (3). In the region of the optimum flow rate, the column also exhibited low relative standard deviations of 4% and 7% for the first moment and plate height, respectively.
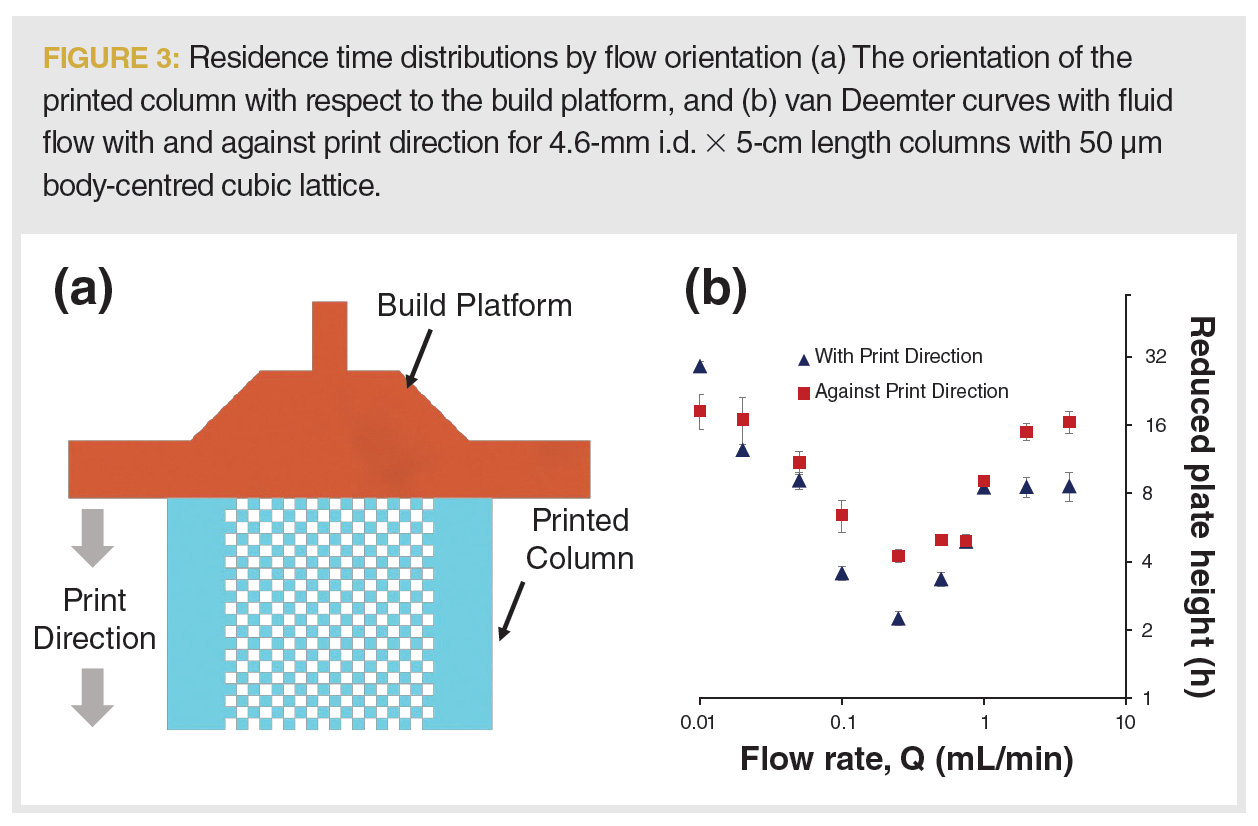
Switching the flow direction of the column resulted in a near‑doubling of plate heights. This difference can be explained by the minor printing artifacts that occur because of a phenomenon called back‑curing, where a small portion at the bottom of a pore in a lattice layer cures and solidifies (10).
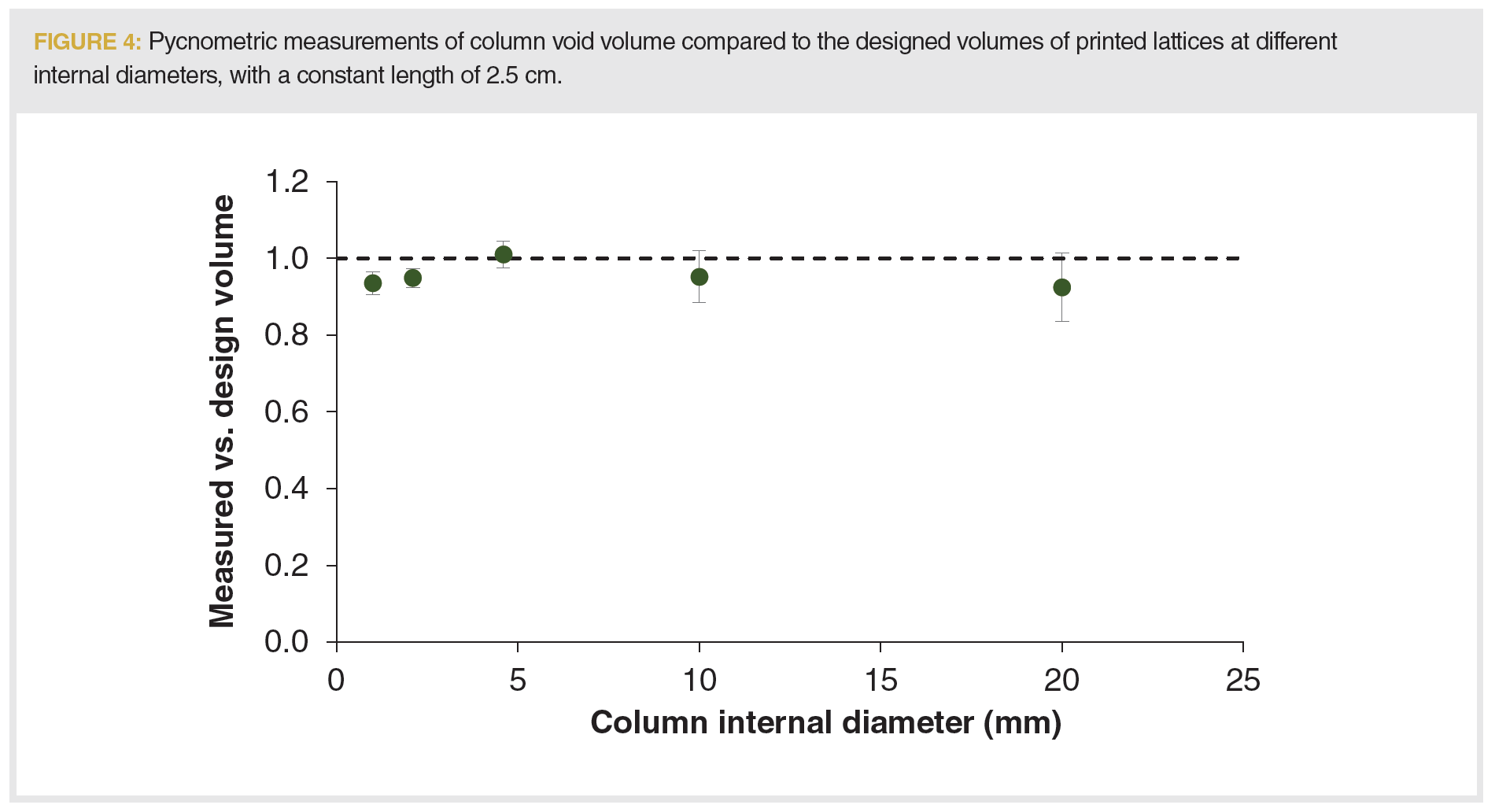
In addition to determining the plate heights, the scalability of the printing method was tested by measuring the void volumes of columns of different internal diameters. Columns with internal diameters of 1–20 mm × 2.5‑cm L were printed in triplicate and measured. As seen in Figure 4, the volume of the printed columns were consistently between 90% and 100% of the design volume, indicating good fidelity between the designed photomasks and the printed structures. However, larger columns (10 and 20 mm i.d.) showed a greater variation in measured void volumes. A possible cause for larger columns showing a greater variation in void volumes is the post‑processing method that was used to remove the uncured resin from the printed lattices. Isopropanol was flushed through the columns using an analytical-scale HPLC system for a fixed column volume. However, to achieve constant flushing velocity, the optimum flow rates for the larger internal diameter columns were significantly higher than the maximum flow rate limits HPLC systems will allow. As a result, the larger printed columns delivered incomplete removal of the uncured resin. Tests with preparative-scale systems are necessary, not just to perform separations on such larger internal diameter columns, but to achieve the desired porosities and void volumes.
Conclusion
This article outlines the development and operation of a novel 3D-printed method, HSLA, geared towards the production of liquid chromatography columns. The method is capable of operating on length scales of micrometres to >40 cm to produce analytical- and preparative-scale column structures in reasonable timeframes, representing a significant improvement compared to existing 3D-printing methods. The characterization of the printed structures show good agreement between the designed and measured void volumes and reduced plate heights as h = 2.25. The results here show that full-scale HPLC and even industrial preparative‑scale 3D-printed columns with an organized internal structure that is consciously designed to fit the application are soon to be a widespread reality.
References
- J.H. Knox, J. Chromatogr. A 960(1–2), 7–18 (2002).
- C. Fee, S. Nawada, and S. Dimartino, J. Chromatogr. A 1333, 18–24 (2014).
- F. Dolamore, S. Dimartino, and C. Fee, J. Chromatogr. A 1532, 150–160 (2018).
- S. Nawada, S. Dimartino, and C. Fee, Chem. Eng. Sci. 164, 90–98 (2017).
- U. Simon and S. Dimartino, J. Chromatogr. A 1587, 119–128 (2019).
- L. Scorza, U. Simon, M. Wear, A. Zouliatis, S. Dimartino, and A. McCormick, Algal Research 55, 102253 (2021).
- W. De Malsche, F. Matheuse, K. Broeckhoven, G. Desmet, D. Cabooter, and S. Eeltink, LCGC Special Issue 36(6), 9–17 (2018).
- S. Nawada, EU patent: EP 19170376.8-1022, 19 April 2019.
- C. Lafratta and L. Li, Three-Dimensional Microfabrication Using Two-Photon Polymerization, Micro and Nano Technologies (William Andrew Publishing, Norwich, New York, USA, 2nd Ed., 2019), pp. 385–408.
- H. Gong, M. Beauchamp, S. Perry, A. Wooley, and G. Nordin, RSC Adv. 5, 106621–106632 (2015).
ABOUT THE AUTHORS
Suhas Nawada is with the Van’t Hoff Institute for Molecular Sciences at University of Amsterdam, in Amsterdam, The Netherlands.
Tristram Budel is with Atum3D, in Gouda, The Netherlands.
ABOUT THE COLUMN EDITOR
David S. Bell is a director of Research and Development at Restek. He also serves on the Editorial Advisory Board for LCGC and is the Editor for “Column Watch”. Over the past 20 years, he has worked directly in the chromatography industry, focusing his efforts on the design, development, and application of chromatographic stationary phases to advance gas chromatography, liquid chromatography, and related hyphenated techniques. His main objectives have been to create and promote novel separation technologies and to conduct research on molecular interactions that contribute to retention and selectivity in an array of chromatographic processes. His research results have been presented in symposia worldwide, and have resulted in numerous peer-reviewed journal and trade magazine articles. Please direct correspondence to: amatheson@mjhlifesciences.com

Identifying PFAS in Alligator Plasma with LC–IMS-HRMS
April 15th 2025A combination of liquid chromatography ion mobility spectrometry, and high-resolution mass spectrometry (LC–IMS-HRMS) for non-targeted analysis (NTA) was used to detect and identify per- and polyfluoroalkyl substances (PFAS) in alligator plasma.










