A Method Transfer from HPLC to UHPLC with Example of Polyphenols
We recently introduced a new series of UHPLC columns with a particle size of 1.6 µm. Here we explain how to transfer an existing HPLC method to a new UHPLC method using polyphenols as an example.
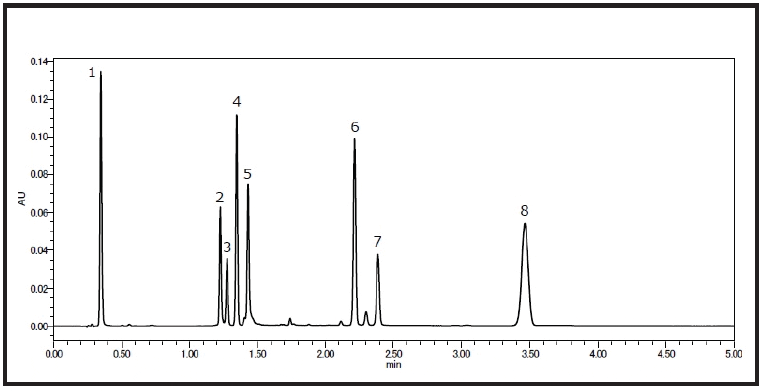
Figure 1: Chromatogram obtained as the first step. Standards: 1. Puerarin 2. Baicalin (37.93)* 3. Resveratrol (2.00) 4. Daidzein (2.75) 5. Quercetin (3.31) 6. Biochanin A (26.69) 7. Curcumin (4.85) 8. Ipriflavone (16.70) *( )s indicate separation factor.
Steps
We previously published an HPLC method on Application Data No. 112 (https://develosil.us/wp-content/uploads/DN112-0519-Analysis-of-Polyphenols-HPLC.pdf). Entering parameters of the method and the specification of the new UHPLC column in a widely available method transfer software program generated an initial gradient table (Table I). The software suggested a flow rate of 0.375 mL/min; this was adjusted to 0.5 mL/min, the optimum flow rate for this column.
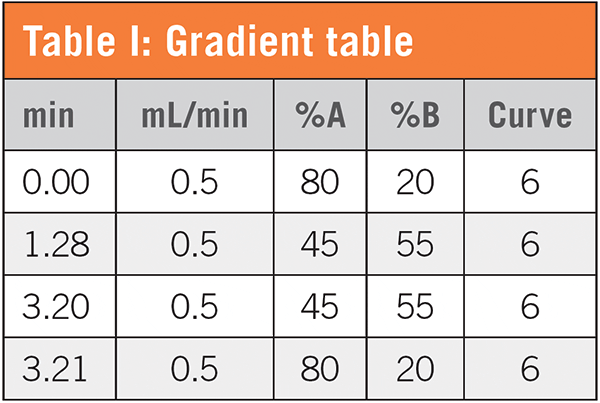
The first thing to decide is the type of detection. If using mass spectrometry, only volatile mobile phase modifiers such as formic acid can be used. In the case of UV detection, acetic acid, formic acid, phosphate buffers, and others can be used. We chose 0.1% formic acid, so that either detection method may be used. Since it can be prepared with a pipette alone, it has the advantages of time and less human error.
The tailing factor and the separation factor obtained using different acids in the mobile phase are shown in Table II for each analyte. Quercetin is known to have a tailing tendency with formic acid, and may also show carryover due to strong ligating properties. Although 0.1% formic acid shows slight tailing with a tailing factor of 1.43, two other mobile phases showed even better results. Considering LC–MS use, we chose 0.1% formic acid as the first candidate. For better peak shapes, 0.08% formic acid + 0.02% TFA is an option.
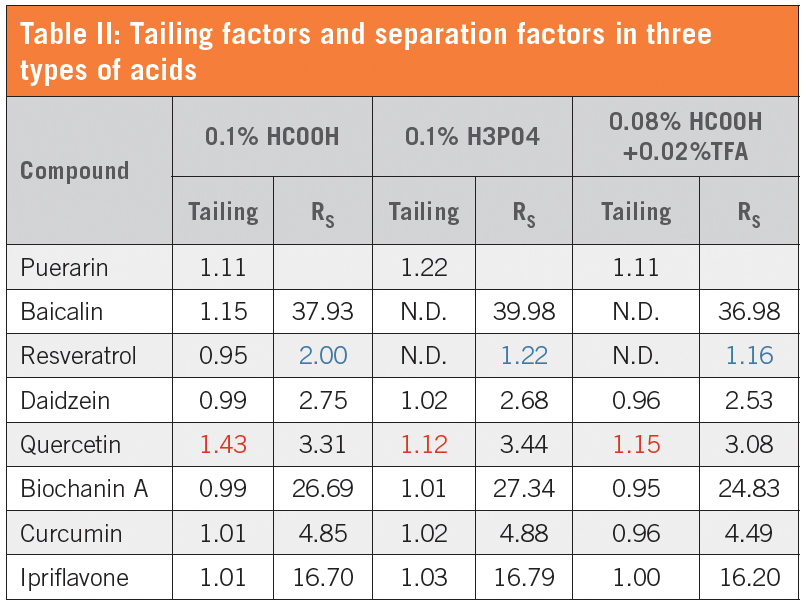
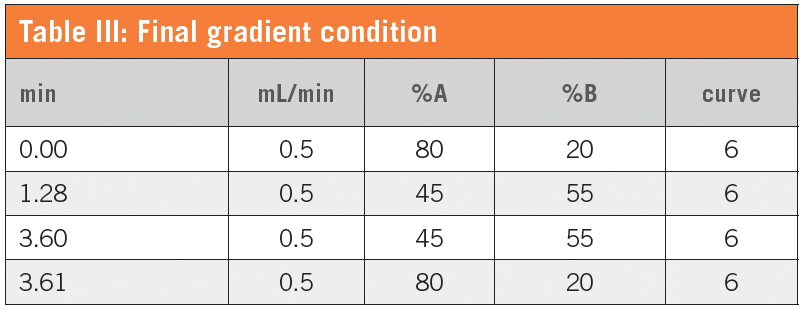
Considering that baicalin and resveratrol have very closely eluted peaks, 0.1% formic acid showed the best separation factor. After adjustments to allow for elution of ipriflavone, we set the final gradient conditions as shown in Table III.
Starting condition
Mobile phase: A) 0.1% formic acid in water, B) 0.1% formic acid in acetonitrile. Conditions: Column: Develosil UHPLC C18, 1.6 µm Size: 2.0 × 50 mm; Temperature: 40 °C; Detection: UV at 260 nm; System: UHPLC with a mixer of 100 µL
Mobile phase: A) 0.1% formic acid in water, B) 0.1% formic acid in acetonitrile

Develosil USA
10060 Carroll Canyon Rd, Suite 100, San Diego CA 92131
Tel and Fax: (858) 800-2433
Website: www.develosil.us

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








