When Electrospray Just Isn't Enough
LCGC North America
In mass spectrometry, the electrospray ionization technique quickly earned its place among the primary analytical tools. Yet it is not always the best tool for the job.
The benefits of ionizing liquid chromatography (LC) effluent using electrospray ionization (ESI) revealed itself early in the 1990s, and practitioners wasted no time urging manufacturers to develop instruments that turned out to be very similar to those we know today. The technique lent itself to the high-throughput, combinatorial chemistry practiced in the pharmaceutical trade. It also worked well for specialties within the chemical industry that depended upon water-soluble analyses and in environmental, food, and clinical laboratories.

Michael P. Balogh
All of which is fine if your analytical target is a relatively polar compound that can exist in solution in an ionized state or can accept a proton as it leaves solution into the gas phase. Indeed, such is the case for many practitioners, so reversed-phase, LC separations are routine in the analytical chemistry field. But many other spectrometrists face a different reality, a chemical diversity that demands various techniques. Some of those techniques were used long before LC–mass spectrometry (MS) became the norm (Figure 1) are well respected in use today. Otherwise, in an LC approach, a seemingly neglected relation of ESI — atmospheric pressure ionization (APCI) — has been the primary resort for analyzing neutral or nonpolar compounds. Recently, another option for less polar samples appeared, atmospheric pressure photoionization (APPI) which, along with laser-assisted ionization (APLI) methods, encompasses many chemical species previously not amenable to ionization by ESI alone. All of which are noted for preserving the analyte intact as it is ionized — in other words, not conferring much direct information other than what is derived from the molecular ion.
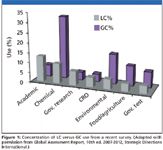
Figure 1
Several alternative ionization techniques whose development had been waiting in the wings have emerged lately as manufacturers accorded them the engineering necessary to merit consideration for daily, robust laboratory use. A few owe their existence to today's unsolved analytical problems, yet they are clearly the extensions of decades-old work.
A niche of LC–MS instrument development that continues to hold our interest is electron ionization (EI). Today's well-accepted ionization modes produce "soft" ionization, which takes place through low-energy chemical processes that yield protonated or deprotonated molecular ions (with or without adducts) but typically, little fragmentation detail. So these modes require MS-MS or high-resolution, accurate-mass MS techniques such as Waters' MSE for analyte characterization. MS-MS observes an ion's transition, caused by colliding with an inert gas under specific energetic conditions, from its molecularly intact state (a precursor ion) paired with a theoretically valid fragment (a product ion). MSE adds accurate-mass determinations to a similar process, using high-energy and low-energy ion spectra to further characterize ions, even in complex matrixes.
Before the advent of current atmospheric techniques, EI enjoyed some limited initial success as the particle beam (PB) interface (1). Nevertheless, the soft ionization techniques displaced it. Despite its limitations — molecular weights must be well under 1000 and candidate compounds must withstand the extremes of high vacuum and high temperature — many, sadly, miss aspects of EI. Among them is its ability to ionize compounds like steroids and polycyclic aromatic hydrocarbons (PAHs), which often are too difficult to ionize by other methods. EI spectra also confer access to well-characterized reference libraries. Spectra are produced largely independent of the design of the source used to obtain the spectrum so libraries offer a wealth of platform-independent, established, interpretable features. Creating reference library spectra in MS-MS can prove difficult because several factors influence the collision ion dissociation (CID) process in a given instrument design. EI provides a direct comparison with the spectra from commercially available electronic resources (National Institute of Standards and Technology [NIST] or Wiley), and makes use of algorithms developed by NIST and Automated MS Deconvolution and Identification System (AMDIS). In the presence of several overlapping peaks, those algorithms extract the analytes' mass spectra in complex chromatographic mixtures.
Achille Cappiello and Pierangela Palma, at the University of Urbino (Urbino, Italy), have continued their interest in what might be considered a modern particle beam (PB) interface. (2). Many from various industries and practices have noted, that one significant shortcoming in quantitative ESI and, to a lesser extent, APCI is the presence of interferences from matrices that induce ion suppression or enhancement (3–5).
Matrix effects can cause unreliable quantitative data (even within a series of replicates), scarce reproducibility, and linearity and accuracy deficiencies in the overall method. They vary with a sample's nature, and the unpredictable response they induce is detectable even among different lots of the same sample or when using the same method. One explanation assumes the analyte and the coeluted matrix can, in the liquid phase, compete for the available charges and, in the gas phase, for the access to the droplet surface (6,7). Ultimately, the remedy is improved sample cleanup procedures and more efficient chromatographic separations. As Cappiello and colleagues point out, novel MS approaches can play a significant role when executed in conjunction with appropriate data analyses. Yet unlike gas chromatography (GC)–MS, in which ionization using 70 eV electrons produces library-searchable spectra for sample identification, soft ionization techniques are not particularly well-suited for analyzing truly unknown compounds.
Aviv Amirav's group at Tel Aviv University, Israel, works with supersonic beams, "cold EI" (8). The beams form when supersonic vibration of a high-pressure liquid spray creates an aerosol that subsequently enters a reduced-pressure expansion chamber. The direct benefit of Cappiello's continued work, however, in addition to a refinement of our understanding of aerosol creation, is its application to a final result. Work from the late 1990s on the very low-flow introduction of a liquid stream into a PB interface demonstrates the extent that PB analyses depend upon the liquid surrounding an analyte of interest. A significantly improved tolerance for nonvolatile buffers was observed, which lead to work with direct EI on an even greater reduced in scale. Pierangela Palma relates:
"The effluent from the column is directly introduced into the ion source at nanoscale flow rates (<500 nL/min) with the advantage to further reduce the adverse effects of mobile-phase vapors. We called it "direct-EI" because the nanocolumn is directly coupled to the MS, with the same simplicity of a capillary GC column (but) without any intermediate apparatus."
In many ways, direct EI models some of the earliest direct-liquid introduction concepts since being reporting in 1974 (9). The typical ion source conditions are high vacuum (about 10-5 Torr) and high temperature. So only a very limited liquid intake can be allowed to enter the ion source.
Unlike the process in atmospheric ionization, an aerosol forms in high-vacuum conditions. The event is followed in quick succession by droplet desolvation and then final vaporization of the solute when it contacts a target surface. The tip of the nebulizer extends into the ion source so that the spray expansion is fully contained inside the volume. The eluate must reach the ion source as liquid, and to prevent a premature, "in-tube" solvent evaporation (a prospect familiar to practitioners of another largely abandoned early 1990s technique called thermospray), the nebulizer and the connecting tubing must be thermally insulated from the source of heat (Figure 2).
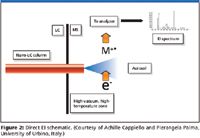
Figure 2
Some Advantages of Very Low Flow Rates
The flow rate used with nanobore columns allows introduction of a steady stream of nonvolatile buffers as a mobile-phase modifier into the ion source. This is welcome news for users of chromatographic separations that benefit from the presence of nonvolatile buffers. In ESI, nonvolatile species cause salt deposits on the metal surfaces that can block ion transmission. Moreover, if an anion and cation pair too strongly with an analyte, the unfavorable competition for charge prevents the analyte of interest from carrying the excess charge on the droplet surface, resulting in reduced sensitivity. For this reason, volatile buffers are the norm in ESI. In the EI ionization mode, however, analytes ionize in the gas-phase independently, and the signal is not influenced by the presence of preformed ions in the form of salts or strong acids and bases.
Cappiello and colleagues explain that in direct EI (as opposed to most API techniques), the gas-phase ionization is a physical-based odd-electron process involving high-energy. Fragmentation occurs spontaneously and pathways are highly reproducible and independent regarding the type of instrument used. Coeluted components ionize independently of each other so that signal intensity invariably relates to the concentration of each analyte. In ESI, the ionization takes place in the liquid phase. So any coeluted substance from the matrix can interfere with the available charges, inducing either ion suppression or enhancement of the signal.
Figure 3 shows a total-ion-current scan profile of 36 substances, at a concentration of 1 mg/mL, almost completely separated by the column. Some analytes were not GC-amenable and gave an uneven response with ESI. For all, Cappiello says it was possible to record interpretable EI mass spectra as well as for three interferences present in the original mixture.

Figure 3
When EI Isn't Enough
Keith Hall, consultant and principal of Hall Analytical Laboratories, Ltd., (Manchester, UK) discovered — as many do — that no single tool solves every problem. An interesting recent investigation among Hall's largely petro-based interests has been of ancient Egyptian mummies. Mummies typify archaeological material in that they represent a finite resource for analyses. To conserve the specimen for future research, analytical methods that maximize information using a minimal amount of sample are essential.
Bitumens play a part in the complex recipes used in the mummification process. Hall found the methods developed by petroleum geochemists for biomarkers analysis and oil–oil correlations apply when determining the presence and origins of archeological bitumens. Says Hall:
"The biomarker analysis of oils typically involves the use of (a) GC (system) coupled to tandem quadrupole instruments (fitted) with EI sources. The main advantage of atmospheric pressure gas chromatography (APGC) I have seen is the ability to obtain accurate mass information on a very wide range of compounds, many of which give poor molecular ion information in traditional EI mode: for instance, unsaturated fatty acids. The technique also gives much higher molecular ions for all compounds in charge transfer mode that I have looked at including n-alkanes. The technique offers the added advantage of information on many compound classes, with the very simple and easy addition of modifiers, to provide protonation. The MS-MS and MSE capabilities are currently being investigated and, when combined with accurate mass information, (they) will provide the chemist with a hugely powerful tool."
Currently, two such devices are offered on the market, one manufactured by Bruker Daltonics, Inc. (Billerica, Massachusetts) and one by Waters Corporation (Milford, Massachusetts). The Bruker offering appeared in a publication claiming compound-dependent limits of detection for a dual purpose LC–MS and GC–MS source of 11.8 to 72.5 nM (10). The Waters offering, based upon work by McEwen (11) and introduced in 2009, couples directly to an existing LC–MS designed for ESI by changing the source housing, a 5-min task much like swapping an ESI probe for an APCI probe. The practitioner then has access to all the software and performance features they have been trained on and rely on in LC–MS but extended to GC as well on the same instrument.
At the heart of the process used with APGC is a plasma generated by a corona pin, much as with APCI, that supplies reagent ions to ionize the target's molecules. Ionization occurs via one of two processes: charge transfer or protonation (Figure 4).
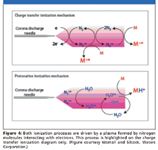
Figure 4
As claimed in a recent white paper on APGC (12), the mass spectra generated by APGC offer unique properties for a number of analytical challenges:
"Field ionization (FI) has been regularly used to generate molecular ion information for compounds which normally fragment when using EI and CI. FI is used due to the "soft" nature of the ionization where little energy is imparted upon the sample molecules, resulting in a significant reduction in bond cleavage. This is observed as a reduction in the degree of fragmentation in the detected mass spectra. FI is a well-established technique; however, it is not without its challenges. It can have a reputation for being difficult to use, requiring a skilled operator with significant experience to maximize its potential."
The authors explain how GC, at atmospheric pressure, can generate mass spectra that evidence some of the qualities of an FI spectrum (dominant molecular ion, reduced fragmentation):
"Using H2O as a modifier produces (a) very clean (M+H)+ base peak, with no fragmentation observed, which could enable identification and confirmation of a structure during structural elucidation studies."
Fragmentation is controlled by reduced field or cone voltage. Nevertheless, if you increase the cone voltage, you can induce a controllable level of fragmentation. Many of the inherent differences and advantages of APGC have been discussed in an earlier article (13).
Surprising Stories from Mummies
From the mummies, Hall took samples of hair and also samples of the coatings covering the linen bandages, coffin, and other funerary items. Sample preparation involved microscale, sealed-vessel (MSSV) analysis of the biomarkers in the asphaltene fraction of the biodegraded bitumens. GC separation was performed (30 m × 0.25 mm, 0.1-μm df DB1-HT column from J&W Scientific), and the eluent from the GC column was swept into a small ion chamber with a supplied make up gas on an APGC-equipped QTOF mass spectrometer (Waters). A plasma from a corona discharge generated reagent ions, which ionized the sample components.
According to Hall, his early results show the atmospheric-pressure GC–TOF-MS system was sufficiently sensitive to detect the biomarkers in the archeological samples. The mummy hair was not subject to previous study. The researchers assumed (incorrectly, it turns out) that it had been treated in the mummification process along with the body (thus ensuring the hair would remain arranged as it had been arranged during life. Hall's examination also identified ancient Egyptian hairstyling products. Using the novel technique soft ionization, compounds below the detection limit of routine GC–quadrupole-MS analysis were obvious immediately. The hair oils, for example, contained mono- and di-alkanoic acids. Compounds at the detection limit of GC-quadrupole MS, such as pentacyclic triterpanes, and steranes, were readily identifiable based upon the enhanced parent ions.
Where hair had been removed by shaving, however, it was treated, like the body, with a mixture of geochemical bitumen, conifer resin, and fats or oils. In addition, the funerary artifacts (for example, coffins and shabti figures), were covered with a black coating. Some coatings covered finely painted polychrome scenes on the coffin. It had not been known whether the coating was black when originally applied or had blackened because of oxidation. The results show that bitumen was a main component of the coatings. Thus, the ancient Egyptians knowingly covered the artifacts with a black coating, a practice that perhaps held significance in the funerary ritual. The subtle differences in biomarker ratios used by petroleum geochemists to determine the source of crude oils also apply to these archeological bitumens.

Figure 5
Hall's examples of petroleum applications demonstrate the direct benefit this renewed, dual technique brings the practitioner. In the spectra derived from the NSO oil standard shown in Figures 5 and 6, note the enhanced molecular ion region and lack of fragmentation normally seen in classical EI, which helps significantly to identify compounds critical for exploration geochemists.
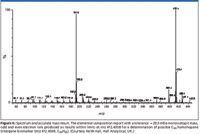
Figure 6
From Hall's early results he states:
"So far, we are observing a tenfold increase in sensitivity for these compounds (using accurate mass) compared to that which we see in EI mode where complex matrices are involved due to the better specificity obtained for the biomarkers over the normal alkanes.
The technique will certainly prove important for petroleum geochemical exploration in the future. The accurate mass identification is a major plus as (will be) the MS-MS mode of compounds of interest for determining the depositional environments of oils and source rocks."
Michael P. Balogh "MS — The Practical Art" Editor Michael P. Balogh is principal scientist, MS technology development, at Waters Corp. (Milford, Massachusetts); a former adjunct professor and visiting scientist at Roger Williams University (Bristol, Rhode Island); cofounder and current president of the Society for Small Molecule Science (CoSMoS) and a member of LCGC's editorial advisory board.
References
(1) R.C. Willoughby and R.F. Browner, Anal. Chem. 56, 2625–2632 (1984).
(2) A. Cappiello, G. Famiglini, P. Palma, E. Pierini, V. Termopoli, and H. Trufelli, Anal. Chem. 80, 9343–9348 (2008).
(3) P.J. Taylor, Clin. Biochem. 38, 328–334 (2005).
(4) W.M.A. Niessen, P. Manini, and R. Andreoli, Mass Spectrom. Rev. 25, 881–899 (2006).
(5) L. Tonidandel and R. Seraglia, Journal of Chromatography Library vol. 72, A. Cappiello, Ed. (Elsevier, Amsterdam, The Netherlands, 2007), pp. 193–210.
(6) N.B. Cech and C.G. Enke, Anal. Chem. 72, 2717–2723 (2000).
(7) R. King, R. Bonfiglio, C. Fernandez-Metzler, C. Miller-Stein, and T. Olah, J. Am. Soc. Mass Spectrom. 11, 942–950 (2000).
(8) A. Amirav and O. Granot., J. Am. Soc. Mass Spectrom. 11, 587–591 (2000).
(9) P.J. Arpino, B.G. Dawkins, and F.W. McLafferty, J. Chromatogr. Sci. 12, 574–579 (1974).
(10) A. Carrasco-Pancorbo, E. Nevedomskaya, T. Arthen-Engeland, T. Zey, G. Zurek, C. Baessmann, A.M. Deelder, and O.A. Mayboroda, Anal. Chem. 81(24), 10071–10079 (2009).
(11) C.N. McEwen and R.G. McKay, J. Am. Soc. Mass Spectrom. 16, 1730–1738 (2005)
(12) Addressing Chemical Diversity And Expanding Analytical Capabilities With APGC, 2010 Waters Corporation, January 2010 720003292en.
(13) M.P. Balogh, LCGC 25(4), 368–381 (2007).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








