Was Moses the First Chromatographer?: Chromatography in the Ancient World
LCGC North America
This month, Leslie Ettre briefly investigates whether the principles of chromatography - either consciously or unconsciously - were used in some ancient work.
Science developed gradually, and usually new developments were based upon some observation of natural phenomena. At first, the observer could not explain it and therefore, often interpreted it as the result of the intervention of some supernatural forces; often centuries passed before the original empirical observation could be explained. If we search the history of mankind and its activities, we can often find such "predecessors" to new inventions.

Leslie S. Ettre
The situation is the same in chromatography. A number of previous columns in this series have pointed out work done before the actual invention of the technique that had been using "chromatographic" principles: I only want to remind my readers about the activities of F.F. Runge, in the middle of the 19th century (1), or D.T. Day and C. Engler in the decades just preceding M.S. Tswett's work (2). But we can go back to ancient times, to the Romans, or even to the Bible and find description of some empirical procedures or tests that a superfluous observer might interpret as resembling chromatography. For example, the general textbook of E. Heftmann (3), quite popular for some time, traced chromatography back to the Moses-led exodus of the Jews from Egypt, and to Pliny the Elder, the great savant of Imperial Rome, living 2000 years ago.
While I would not consider the quoted works as "chromatography," I felt that the readers of LCGC might be interested to know a little more about these and the capabilities of our distant ancestors to use natural phenomena to their advantage.
Was Moses the First Chromatographer?
The Bible deals in details with the exodus of the Jews from Egypt and their long wandering in the wilderness before finally reaching the land promised them by God. Among others, we can find the following narrative (4):
So Moses brought Israel from the Red Sea, and they went out in the wilderness of Shur; and they went three days in the wilderness, and found no water.
And when they came to Marah, they could not drink of the water of Marah, for they were bitter; therefore the name of it was called Marah.
And the people murmured against Moses, saying, What shall we drink?
And he cried unto the Lord and the Lord shewed him a tree, which when he had cast into the waters, the waters were made sweet.
Figure 1 shows the most likely route of the wandering of the Jews, indicating the possible location of "Marah." It is a Hebrew word meaning bitterness, and local people still refer to the bitter waters of this area as Al Buhayrat al Murrah. Evidently the existence of certain shrubs in that area had been known which could be used to sweeten bitter water: this is mentioned by J.H. Hertz in his commentaries to the Pentateuch (5). Thus, Moses most likely used this observation to solve their critical problem.
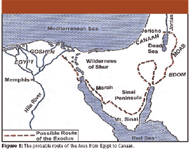
Figure 1
Using our present knowledge we might interpret Moses' miracle as ion exchange, thus, we might conclude that Moses used a kind of ion-exchange chromatography. It should, however, be mentioned that in "chromatography" we have a flowing stream, while the water of Marah was most likely stagnant. Thus, it is a matter of interpretation whether we consider Moses as the first chromatographer!
Did Pliny the Elder Use Planar Chromatography?
Gaius Plinius Secundus (23–79 A.D.) (Figure 2) was the scion of a high-ranking Roman family who had high government posts in Imperial Rome, has served in the military, and traveled widely in Roman-occupied Gallia, Spain, Germany, and North Africa. He was a well educated man who continuously studied nature and the foreign lands, and wrote numerous books summarizing his findings. In 77 A.D., Pliny was appointed the commander of the fleet in the Bay of Naples: He was there in 79 A.D. when Mount Vesuvius erupted, burying Pompeii and Herculaneum. Pliny went ashore to study closely the catastrophe, and there he died through inhaling the poisonous gases emitted by the volcanic activity.

Figure 2
Pliny the Elder wrote a number of books; probably the greatest of his works is the Historia Naturalis ("Natural History"). This is an encyclopedic work encompassing all the knowledge of nature and science as known during Roman times: description of the world, geography, ethnography, anthropology, zoology, botany and agriculture, pharmacology, mineralogy, and art. In the Middle Ages, Pliny's work was considered as the authoritative compilation of knowledge, regardless of whether the discussion was based upon observation and facts, or was simply pure fiction, reflecting even superstition.
The Historia naturalis is divided into 37 libri ("books"). Pliny the Elder published the first 10 books by himself in 77 A.D., and was preparing the rest for publication when he died. Upon his sudden death, his nephew, Pliny the Younger (63–ca.113 A.D.), has served as the executor of his will and the organizer of his literary remains, publishing also the rest of this work.
Two subjects in Pliny's work are sometimes related to what we call today planar chromatography: testing the purple dye used by the Roman upper class to dye the border of their toga, and checking whether verdigris is not adulterated. Pliny's full work is available in a modern bilingual (Latin–English) edition, translated and edited by H. Rackham (6). Thus, I could study the pertinent passages.
The purple dye was extracted by the Romans from the fish purpura (sea purple) by a complex process well described by Pliny (7), starting with the way how to catch the fish, up to its testing. Historical discussions of chromatography sometimes mention this as spotting the dye on a piece of cloth and observing the development of the color, and interpret it as a forerunner of paper chromatography. However, authors of this reference probably never read Pliny's original writing: what he actually suggested was to dip a piece of clean fleece in the aqueous dye extract and observe the intensity of the color. In other words, it was simply a dyer's test, and has nothing to do with chromatography.
The second test often referred to is related to verdigris. Verdigris (vert de Grèce or green of Greece; in Latin aeruginis) was prepared by the Romans by reacting copper and strong vinegar (forming copper acetate) and used as medicine. (Today the expression verdigris is also used for the greenish patina formed on copper, brass, or bronze surfaces exposed to the atmosphere. However, that is chemically different: it is basic copper sulfate.)
Evidently verdigris had been a popular remedy in Rome and was often adulterated; therefore, Pliny described ways how it could be tested (8). It is worthwhile to quote here the pertinent passage (my notes in brackets):
Rhodian verdigris is adulterated chiefly with pounded marble, though other use pumice-stone or gum. These adulterations can be detected by the teeth as they crackle when chewed. . . . But the adulteration of verdigris that is the most difficult to detect is done with shoemakers' black [this is iron sulfate]. . . . Shoemakers' black can be detected by means of a papyrus previously steeped in an infusion of plantgall [containing tannic acid]: when smeared with genuine verdigris it turns at once black.
This is certainly a chemical test and is based upon the reaction of copper with tannic acid. However, again, it is not chromatography: what happens is not separation, but reaction on a medium, but interestingly, it is also somewhat similar to the work of Runge 1800 years later, who also carried out reactions on pieces of filter paper, and close to the Tüpfelanalyse (spot tests) developed by Fritz Feigl in the 1930s. Thus, none of the tests mentioned by Pliny had anything to do with chromatography.
We can conclude that our ancestors were keen observers of natural phenomena and developed empirical tests based upon such observations. However, we should not artificially make conclusions, with interpretations based upon our present-day knowledge.
Acknowledgment
Figure 2 was taken from Botany Online, an Internet text compiled by the late Professor Peter von Sengbusch (Universities of Bielefeld and Hamburg).
From 1988 to 2004, "Milestones in Chromatography" editor Leslie S. Ettre was associated with the Chemical Engineering Department of Yale University (New Haven, Connecticut), first as an adjunct professor and then as a research fellow. Previously, he had been with the Perkin-Elmer Corporation for 30 years. He is currently a member of LCGC's editorial advisory board. Direct correspondence about this column to "Milestones in Chromatography," LCGC, Woodbridge Corporate Plaza, 485 Route 1 South, Building F, First Floor, Iselin, NJ 08830, e-mail lcgcedit@lcgcmag.com.
References
(1) H.H. Bussemas and L.S. Ettre, LCGC 22(2), 262–270 (2004).
(2) L.S. Ettre, LCGC 23(12), 1274–1280 (2005).
(3) E. Heftmann, in Chromatography — a Laboratory Handbook of Chromatographic and Electrophoretic Methods, 3rd ed., E. Heftmann, Ed. (Van Nostrand Reinhold, New York, 1975), pp.1–13.
(4) Exodus, Chapter 15 §22–25 (King James Version).
(5) The Pentateuch and Haftorahs, 2nd ed., J.H. Herz, Ed. (Soncino Press, London, UK, 1977), pp. 273–274.
(6) H. Rackham (editor and translator), Pliny: Natural History, with an English Translation in Ten Volumes (Harvard University Press, Cambridge, Massachusetts, 1938–1963).
(7) Pliny the Elder, Historia Naturalis, Book IX, chapters LXI–LXIII, pp. 126–137.
(8) Pliny the Elder, Historia Naturalis, Book XXXIV, chapters XXV–XXVI, pp. 108–113.
Maximizing Cannabinoid Separation for Potency Testing with LC
April 7th 2025Researchers from the Department of Chemistry at Western Illinois University (Macomb, Illinois) conducted a study to optimize the separation of 18 cannabinoids for potency testing of hemp-based products, using liquid chromatography with a diode array detector (LC–DAD). As part of our monthlong series of articles pertaining to National Cannabis Awareness Month, LCGC International spoke to Liguo Song, the corresponding author of the paper stemming from this research, to discuss the study and its findings.
How Many Repetitions Do I Need? Caught Between Sound Statistics and Chromatographic Practice
April 7th 2025In chromatographic analysis, the number of repeated measurements is often limited due to time, cost, and sample availability constraints. It is therefore not uncommon for chromatographers to do a single measurement.












