Ultra-Fast Analysis of Alkaloids and Permitted Additives in Dark Chocolate Products
A simple, rapid, and robust UHPLC method for the simultaneous determination of polyphenols, methylxanthines, sweeteners, and flavouring substances as well as some common preservatives has been developed using an automated method scouting and method optimization workflow.
A simple, rapid, and robust ultrahigh-pressure liquid chromatography (UHPLC) method for the simultaneous determination of polyphenols, methylxanthines, sweeteners, and flavouring substances as well as some common preservatives has been developed using an automated method scouting and method optimization workflow. The most suitable mobile phase and stationary phase combination was identified in an overnight scouting run. These conditions were used to create a two-dimensional model using computer simulation software. Temperature and gradient time were varied to establish the best separation conditions. This approach resulted in a 1.5 min gradient method that could screen 17 compounds of interest.
PHOTO CREDIT: MALERAPASO/GETTY IMAGES

Chocolate is one of the most popular indulgences available. As well as the well-known negative effects of any high-fat, high-sugar food product, there is also evidence of health benefits related to chocolate consumption. These positive effects, such as lowered blood pressure, antioxidant properties, reduction in the risk of cardiovascular disorders, and an increase in cognitive abilities, are all attributed to the flavonoid content, which is only relevant in dark chocolate products (≥ 40% cocoa) (Flavonoids can also be found in milk chocolate but in a very low concentration).
However, a high cocoa and a high flavonoid content comes with an even higher amount of less desirable alkaloids, namely the stimulants caffeine and theobromine. In addition to the natural products found in cocoa beans, a large number of permitted additives can be added to chocolate products to alter the taste, texture, or expiry date, such as artificial or natural sweeteners, and flavouring compounds or preservatives. To monitor the composition and therefore the quality of cocoa in the food product, an analytical method needs to enable individual quantification of any of these potential ingredients.
This article describes the development and optimization of a rapid ultrahigh-pressure liquid chromatography (UHPLC) screening test for the separation and quantification of polyphenols, methylxanthines, sweeteners, and flavouring substances as well as some common preservatives for the quality control of dark chocolate products.
Experimental
Equipment and Chromatographic Methods: For UHPLC method scouting, a Shimadzu Nexera X2 Method Scouting System was used, consisting of two quaternary solvent pumps, autosampler, and column oven, including a six-column switching valve. The system was also equipped with a high resolution UV photo diode array (PDA) detector and an LCMS-8040 triple quadrupole mass spectrogmeter via an electrospray ionization (ESI) source.
Method scouting was performed in an overnight sequence using 5 min gradient runs at 40 °C. Five combinations of stationary phase and mobile phase were selected. The columns comprised a variety of bonded phases that exploit multiple mechanisms of separation and therefore provide alternative selectivity to common C18 phases. The aqueous and organic mobile phase combinations contained the same buffer salt/acid (AA/BA; AB/BB; and AC/BC).
Figure 1: Colour-coded resolution map for UHPLC method development.

After method scouting a column with a polar embedded group for enhanced polar selectivity was found to offer the best separation from the columns selected with all analytes of interest at least partly separated using a mobile phase consisting of A: 10 mM ammonium formate in H2O (pH 2.8) and B: 10 mM ammonium formate (pH 2.8) in MeCN/H2O (90:10 v/v). These conditions were used to create a two-dimensional DryLab model (Molnár Institute) using 2 min and 6 min gradient runs at 25 °C and 50 °C as input data. These experiments resulted in a colour-coded resolution map for simple identification of the optimum separation conditions (Figure 1).
The software predicted an optimum separation with a minimum resolution of the critical peak pair of 1.6 in a gradient run from 5% to 85% B in 1.5 min at a flow rate of 1.2 mL/min at 42 °C.
Figure 2: Comparison of the predicted and actual chromatogram of the UHPLC analysis of alkaloids and permitted additives in dark chocolate products.

To obtain the best possible resolution the column was packed with 2-µm particles instead of the 3-µm particle size tested initially. The selectivity of the column was identical and the pressure increase was not higher than predicted by the software so the change in particle size did not cause any problem for the separation. A comparison of the predicted chromatogram with an actual sample run is displayed in Figure 2.
Sample Preparation: The chocolate sample was defatted and the compounds extracted into a sample solvent suitable for high performance liquid chromatography (HPLC) analysis.
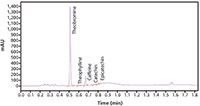
Figure 3: UHPLC analysis of the extracted chocolate sample in a 1.5 min gradient with PDA detection. Column: ACE Excel 2 C18-Amide, 100 x 2.1 mm, mobile phase: 10 mM HCOONH4, pH 2.8 in A: H2O and B: MeCN/H2O (90:10 v/v), 1.2 mL/min flow, 42 °C.
A 2 g sample of dark chocolate (≥ 63% cocoa) was spiked with approximately 100 µg of standard substances before being defatted twice with 15 mL of n-hexane for 10 min in an ultrasonic bath at 30 °C. The mixture was then centrifuged for 10 min at 6000 rpm. The supernatant with the dissolved fat was discarded and the precipitate was air-dried and reconstituted in a mixture of acetone, water, and acetic acid (70:29.8:0.2 v/v/v) and sonicated for another 15 min at 30 °C. The sample was then filtered and heated to 40 °C to evaporate the organic solvent. The remaining aqueous fraction was filtered again and used for UHPLC analysis.
Results of the LC–UV and LC–MS Analysis
Figure 4: LC-MS analysis of the extracted chocolate sample in a 4.5 min gradient. Column: ACE Excel 2 C18-Amide, 100 × 2.1 mm, mobile phase: 10 mM HCOONH4, pH 2.8 in A: H2O and B: MeCN/H2O (90:10 v/v), 0.4 mL/min flow, 42 °C.
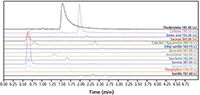
In Figure 3, the results of the analysis of an extracted, spiked chocolate sample are displayed. With PDA detection the methylxanthines theobromine, theophylline, and caffeine as well as the flavonoid content of catechin and epicatechin were determined. SIM detection combined with mass spectrometry (MS) via an ESI source also highlighted the presence of sucrose, which can not be detected with an absorbance detector, and trace amounts of sorbic acid, ethyl-vanillin, vanillin, quercetin, acesulpham, and saccharin that were spiked into the chocolate sample to test the extraction method. When using MS detection the flow rate was reduced to 0.4 mL/min and gradient time increased to 4.5 min.
Conclusion
A robust, fast, and sensitive UHPLC/LC–MS method for the simultaneous separation of polyphenols, methylxanthines, sweeteners, flavouring substances, and preservatives in dark chocolate products has been developed. The method scouting experiment and optimization using computer simulation software was able to save time and offered visualization of the design space in a resolution map to establish the most robust separation method. Adaptation to highly sensitive LC–MS detection was feasible using the ESI source.
Brigitte Richrath graduated with a diploma in chemistry from the Heinrich-Heine-University in Düsseldorf, Germany, in 2007 and was awarded her doctorate for research in (metal-) organic chemistry and stereoselective synthesis in 2010. In 2011, she started working as a HPLC product specialist with Shimadzu Deutschland, and since 2013 has been employed in the analytical business unit of Shimadzu Europa in Duisburg, Germany.
Gesa Johanna Schad graduated with a diploma in chemical engineering from the technical University, NTA in Isny, Germany, in 2004 and as a Master of Science in pharmaceutical analysis from the University of Strathclyde in Glasgow, UK, in 2005. Until 2006, she worked as a consultant in HPLC method development and validation in an analytical laboratory of the FAO/IAEA in Vienna, Austria. She gained her doctorate for research in pharmaceutical sciences at the University of Strathclyde in 2010 and was employed as an HPLC specialist in the R&D department at Hichrom Ltd. in Reading, UK, from 2009. Since 2013, she has worked in the analytical business unit of Shimadzu Europa in Duisburg, Germany, as an HPLC product specialist.
E-mail: shimadzu@shimadzu.eu
Website: www.shimadzu.eu/
This article is from The Column. The full issue can be found here: http://www.chromatographyonline.com/vol-10-no-11-column-june-19-2014-europe-and-asia-pdf

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










