The LCGC Blog: The Practicalities of Dead Volume Optimisation in UHPLC
I’ve previously written on ‘Putting the U is your UHPLC’ where I highlighted several aspects of working optimally with high pressure liquid chromatography (1), especially with regards to system chromatographic efficiency (N). In this instalment, I wanted to talk a little more about the instrument factors that need to be considered when optimising your UHPLC system to match the column hardware and analytical requirements for your applications. Why is this important? Well at some point, our drive for higher efficiency, faster chromatography, and the spacing between peaks (i.e. the resolution) will be reliant not only on the selectivity of the separation, but increasingly importantly on the peak width (i.e. the efficiency).
As a preamble, I would point the reader to the excellent recent LCGC Troubleshooting series on Extra Column Volume and its relationship with chromatographic efficiency written by Dwight Stoll, Thomas Lauer, and Ken Broeckhoven (2). This series of four articles takes a deep dive into many of the topics that I’m about to explore and I would thoroughly recommend that readers refer to the series for further information. Secondly, I have used two excellent recent resources to illustrate the examples in this article. The first is an LC Simulator/Teaching aid by Davy Guillarme, Balazs Bobaly, and Jean-Luc Veuthey of the School of Pharmacy, University of Geneva, Switzerland (3), the second is a very detailed extra column volume effect (dispersion) calculator developed by Dwight Stoll, Thomas Lauer, and Ken Broeckhoven (4). Whilst these software tools are often seen as only being of use within the classroom, I can attest that I’ve often used tools of this type to better analyse and understand important relationships in HPLC and to troubleshoot issues that I’m having in the laboratory. I hope I can highlight their usefulness below.
In understanding the implications of extra column volume on dispersion of the analyte band and therefore on chromatographic efficiency, one needs to understand the factors and system components which impact dispersion and therefore the efficiency of the analyte peaks obtained.Figure 1 illustrates the system components which contribute to dispersion of the analyte from the point of injection to the point of detection.
Figure 1: System Components to be considered in any assessment of dispersion within an HPLC system (Reproduced with permission from Reference 1).
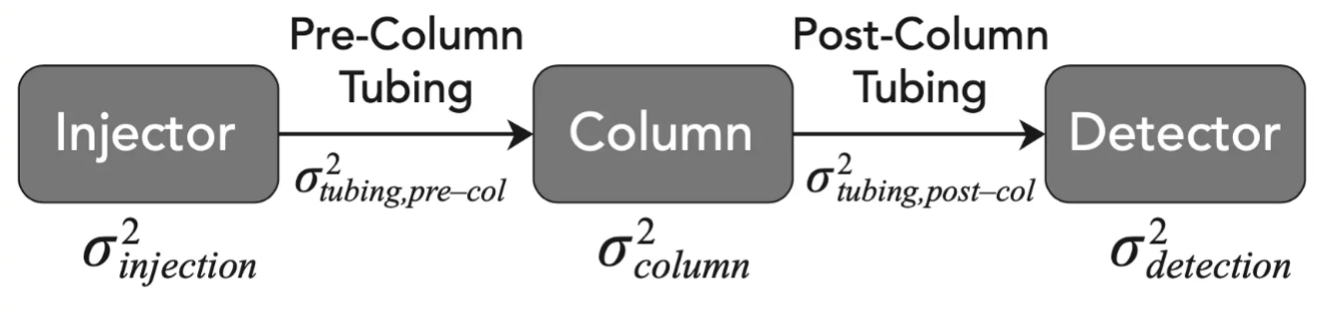
I’ve listed below the components and variables which can have a direct effect on system dispersion, and which need to be considered during any optimisation exercise:
- Mode of chromatography (isocratic versus gradient)
- Diffusivity of the analytes in the solvents used
- Viscosity of the eluent
- HPLC column dimensions and packing material characteristics (particularly the porosity of the packing material)
- The eluent flow rate
- Injector tubing internal dimensions
- Fixed loop internal dimensions
- Variable loop dimensions and the volume of any needle and seat components
- The injected sample volume
- Tubing internal dimensions between the autosampler and the column compartment heat exchanger
- The internal volume of any eluent pre-heater within the column compartment
- The internal volume of the HPLC column
- The internal dimensions of the tubing joining the column to the detector and/or
- The internal dimensions of any flow splitter used
- The internal dimensions of any tubing used post splitter to connect to the detector
- The internal volume of the detector flow cell and tubing
- The sampling rate and bunching rate (often known as the Time Constant) of the detector
I think we would all agree that this is a very long list of considerations! This is precisely where software tools can be so useful. The Dispersion Calculator of Reference 4 is an excellent resource to help prompt the user for the required information and provides an excellent summary of the factors which contribute most to analyte peak dispersion. A screenshot of the tool is shown in Figure 2.
Figure 2: A useful software tool for assessing dispersion within HPLC (2,4).
To illustrate the importance of various contributors to extra column volume, and to illustrate some of the typical values for volumes of each of each of the contributing components, I will use illustrative examples of a ‘typical’ UHPLC system, a UHPLC system which has been optimised for low dispersion, a system which includes a split for the use of both UV and MS detection and a system where the detector signal acquisition and processing parameters have not been correctly optimised.I often find that users are most often dissuaded from any system dispersion computations because the information on system volumes are difficult to obtain from instrument manufacturers or reference literature.Again, the tool from Reference 4 is very helpful in also suggesting ‘typical’ values or explaining the derivation of the required information.
Application Details:
Sample:Lidocaine formulation
Eluent: Isocratic, 60% 20mM phosphate buffer (pH 7.0), 40% Acetonitrile
Flow Rate: 0.5 mL/min
Injection Volume: 0.5 mL
Sample Diluent: 80% Water: 20% Acetonitrile
Column: C18 50 x 2.1 mm, 1.7 mm, porosity 0.55
Column Temperature: 35oC
Detection:UV @ 220nm, Acquisition rate and time constant variable
For this separation I have used several model UHPLC systems, the first of which is a highly optimised, low dispersion system outlined in full detail below and the output from the Dispersion Calculator is shown in Figure 3.
Mobile phase viscosity (cP): 0.76 (Important in estimating system back pressure (5))
Needle seat diameter [µm]: 110
Needle seat length [mm]: 100
Tubing (pre-column):
Diameter [µm]: 80
Length [mm]: 220
Column Compartment Heat exchanger and capillary:
Diameter [µm]: 75
Length [mm]: 250
(one might also use the published value of the heat exchanger unit as these values are often more readily available from manufacturers)
Tubing (post column):
Diameter [µm]: 80
Length [mm]: 220
Detector flow cell:
Volume [µl: ]0.6 Dispersion co-efficient (ꝋ): 0.44
Analyte Retention Factor: 2.29 (see later for further explanation)
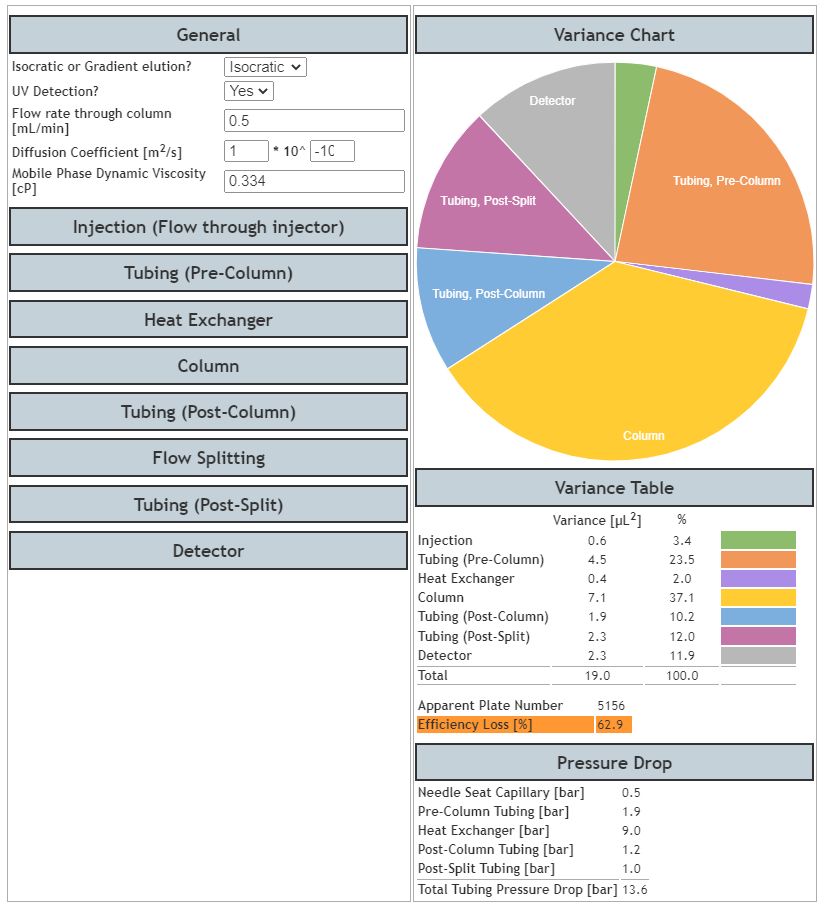
From Figure 3 one can see that the majority of the dispersion is occurring within the column, which in a high performance HPLC system, is as it should be. In total, only 28% of the theoretical efficiency which could be derived by this system — including the column — is lost due to dispersion effects, with the extra column dispersion effects evenly balanced across all components with no dominant contributor to dispersion (Variance).
Figure 3: Output from the Dispersion Calculator for the Dead Volume Optimised ‘Model’ UHPLC system
However, we must be realistic, and given that we are using UHPLC systems primarily for speed as well as peak capacity, we should consider the efficiency gains or losses for peaks across the chromatogram. That is, efficiency loss, and the contributing factors, can differ depending upon the retention factor of the analyte of interest.
In order to visually assess the efficiency changes, the example separation I have used is included in the excellent HPLC Simulator of Reference 3. A screenshot of the simulator programmed with some of the values above as well as an expansion of the resulting chromatogram is shown in Figure 4.
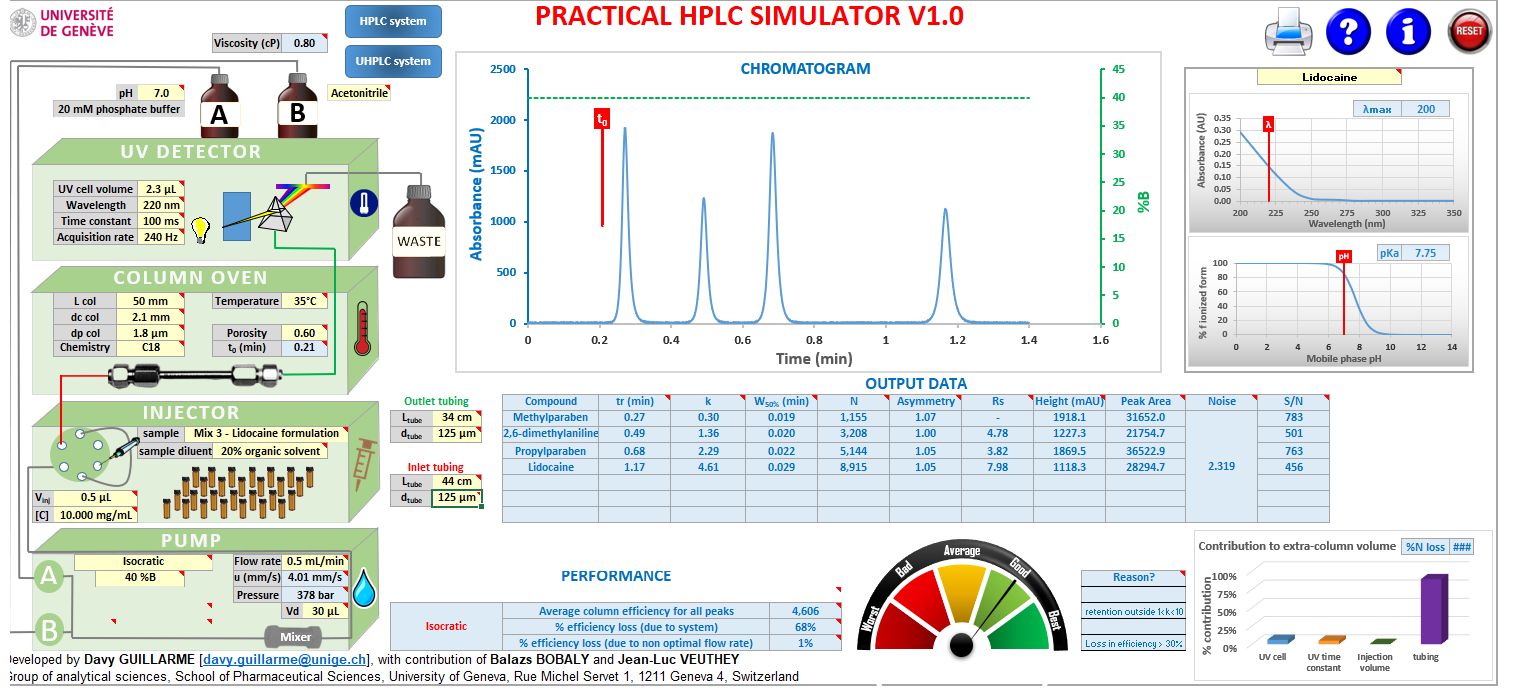
Figure 4: Chromatogram generated by the HPLC Simulator of Reference 3 and the chromatogram resulting form the highly optimised model UHPLC system of Figure 3.
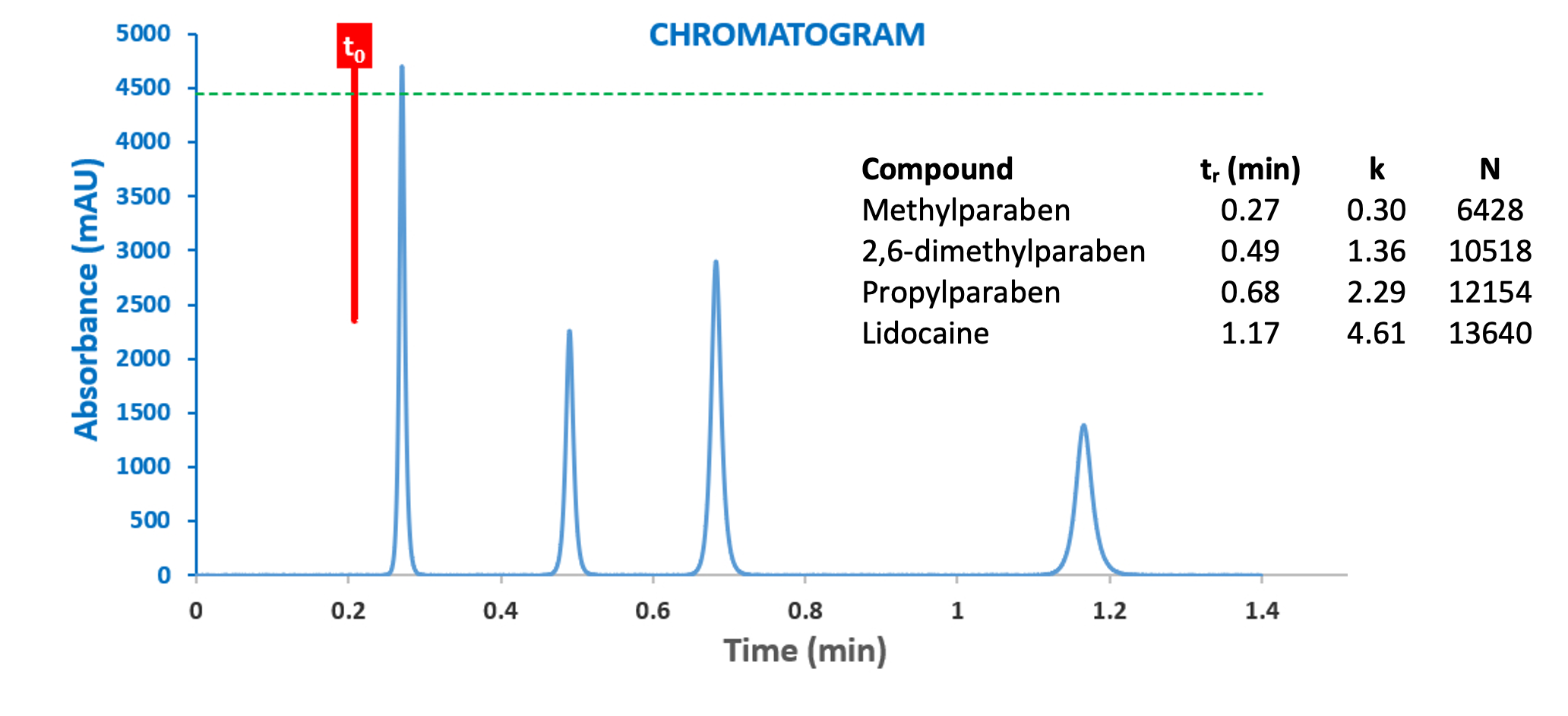
The Simulator allows us to input most of the system variables which are used in the Dispersion Calculator but also allows the eluent variables to be entered in order to generate the simulated chromatogram. Figure 3 calculated the dispersion variances for the propylparaben peak (k = 2.29), but what would these results look like if we calculated for the very early eluting methylparaben peak (k = 0.3)?
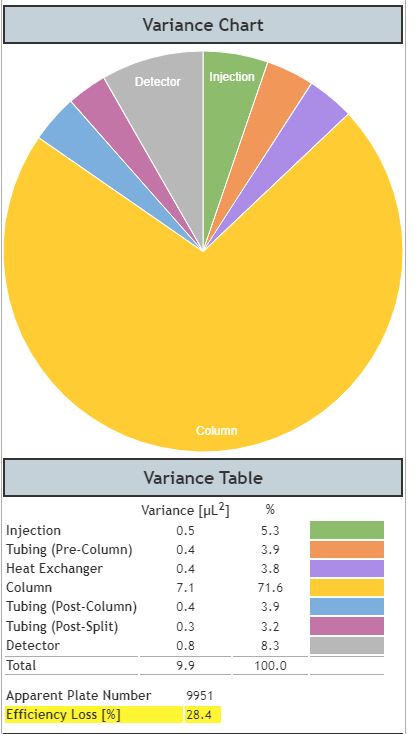
Figure 5: Dispersion in a dead volume optimised UHPLC system when considering an analyte with low retention factor (k=0.3).
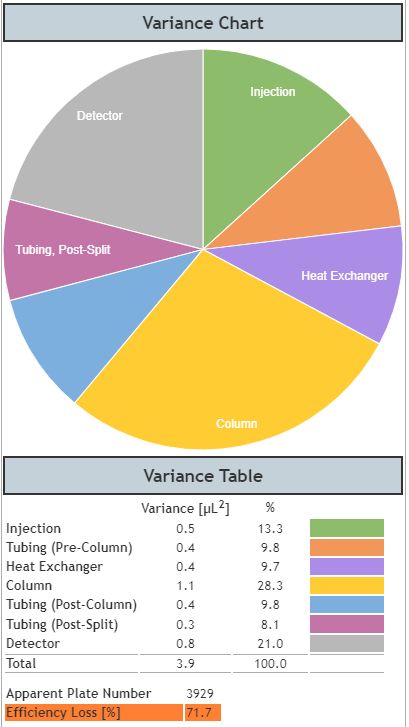
From Figure 5 we can see that, even though the peak looks ‘sharp’ to a chromatographer’s eye, it is only achieving around 30% of the theoretical efficiency of the system and the other system components are now contributing a significantly higher proportion of the overall variance. However, the low retention factor is also influencing the overall system variance with the column variance now being 1.1 as opposed to 7.1 for the propylparaben peak which has a significantly higher retention factor, and therefore suffers from increased on-column dispersion. This example serves to highlight the importance of extra-column dispersion on early eluting components in UHPLC.
If the post column effluent requires to be split, for example to enable both UV and MS detection to be operated in parallel, then the splitter and post column tubing may be a significant further contributor to band broadening. Not only does the extra tubing contribute modestly to peak dispersion, but the volume of the analyte exiting the splitter is very small and therefore is highly susceptible to dispersion processes within the post-split tubing and detector.
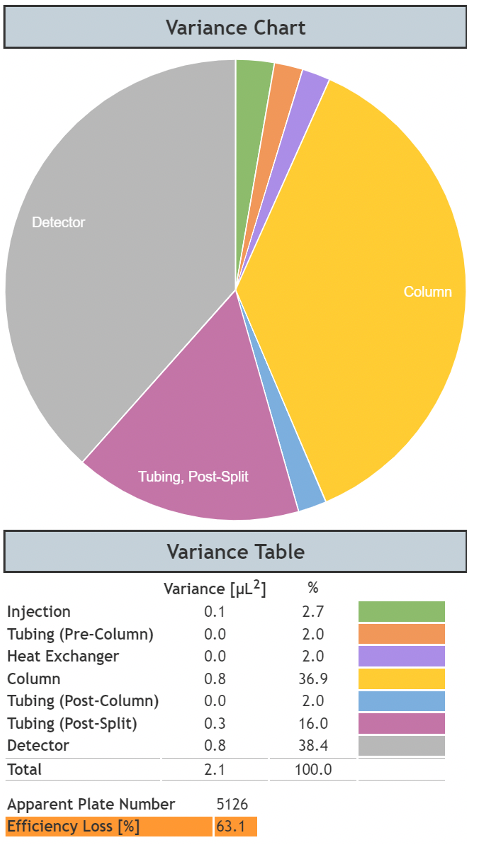
Figure 6 shows the results of a split of 1:2 and post column tubing of 80 mm x 220mm. The propylparaben peak which suffered only approximately 30% loss of efficiency versus the theoretical maximum now loses approximately 60% of its theoretical efficiency, simply by introducing a 2:1 split and associated post-split tubing.
Figure 6: System dispersion contribution in an ‘ultra-low’ dispersion UHPLC system with a post-column split of 1:2.
So now we have seen what might be achieved with a highly optimised UHPLC system, what might we expect from a ‘standard’ UHPLC system which has not been optimised for low extra column dispersion.
Needle seat diameter [µm]: 120
Needle seat length [mm]: 100
Tubing (pre-column):
Diameter [µm]: 120
Length [mm]: 340
Column Compartment Heat exchanger and capillary:
Diameter [µm]: 120
Length [mm]: 250
(one might also use the published value of the heat exchanger unit as these values are often more readily available from manufacturers)
Tubing (post column):
Diameter [µm]: 120
Length [mm]: 220
Detector flow cell: Volume [µl: ]1.0, Dispersion co-efficient (ꝋ): 0.44
Analyte Retention Factor: 2.29
Figure 7: System dispersion contributions and the resulting chromatogram from a Lidocaine Preparation derived using a model UHPLC system with ‘Standard’ dispersion.
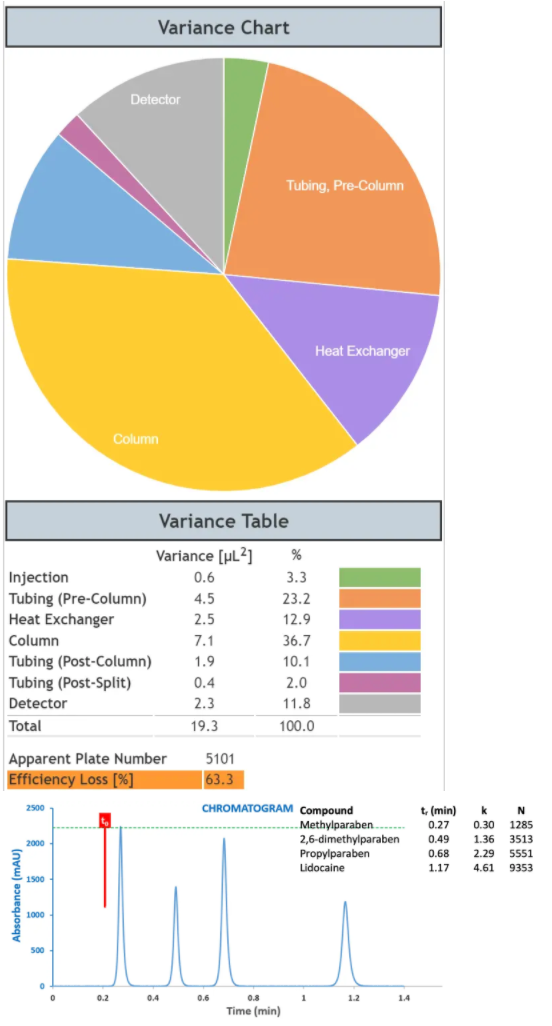
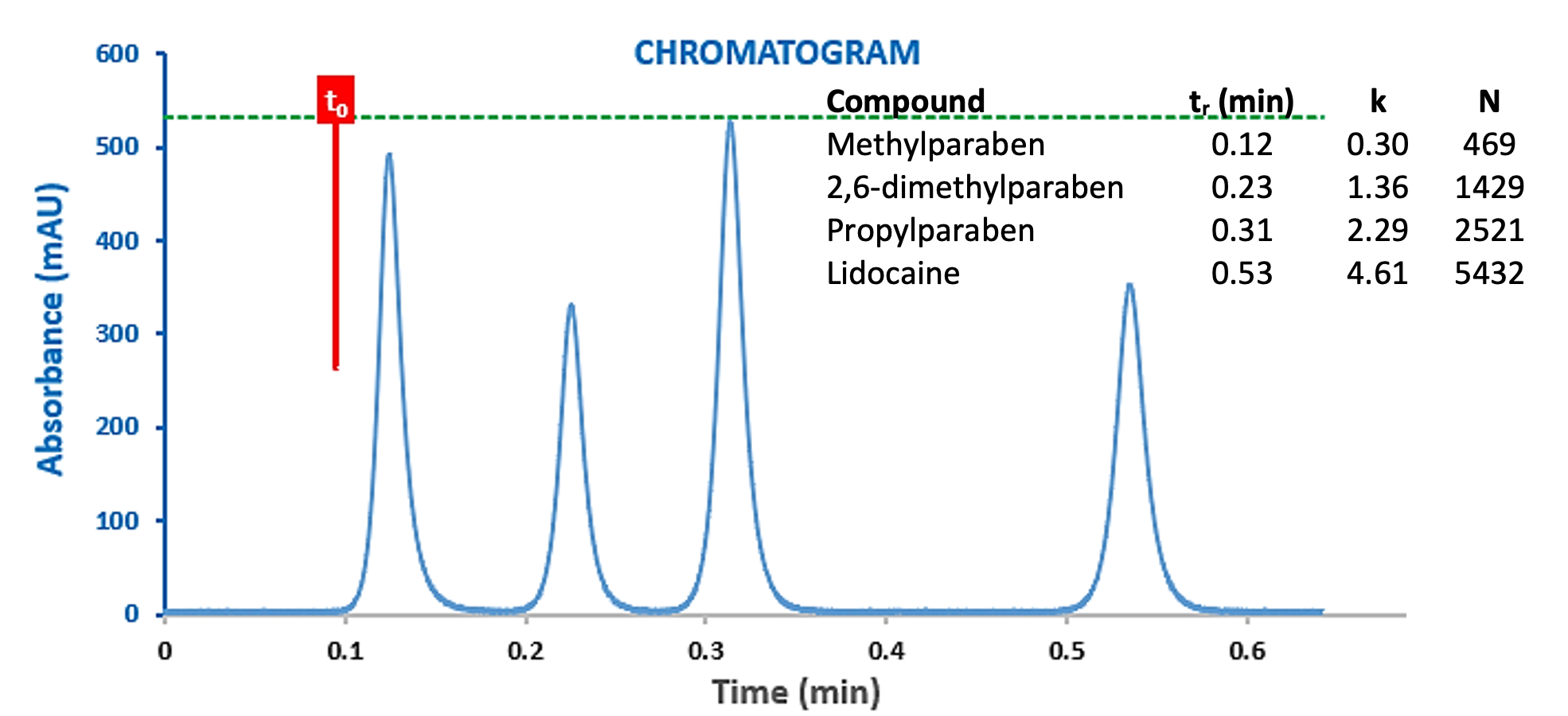
As can be seen from Figure 7, the ‘standard’ UHPLC system shows around half of the efficiency of the dispersion optimised system, that is, the efficiency loss versus the theoretical value are around 60% and the total system variance (dispersion) is approximately twice that of the optimised system (19.1 versus 9.9 mL2). The efficiency of the propylparaben peak has reduced from 12154 plates to 5551. If we were to introduce a post column split of 1:2 with a 220mm x 120 µm capillary into this system, then the propylparaben peak would suffer a loss of 87% loss of efficiency against the theoretical value! Whilst the separation under consideration here (Figure 8) would remain viable under these conditions, more complex separations may risk loss of resolution and the system will be operating sub-optimally. Interestingly, Figure 8 also shows the same separation at a flow rate of 1.0mL/min, which demonstrates the effects of increased eluent flow rate (1.0 mL/min) which can exacerbate the dispersion post flow splitting.
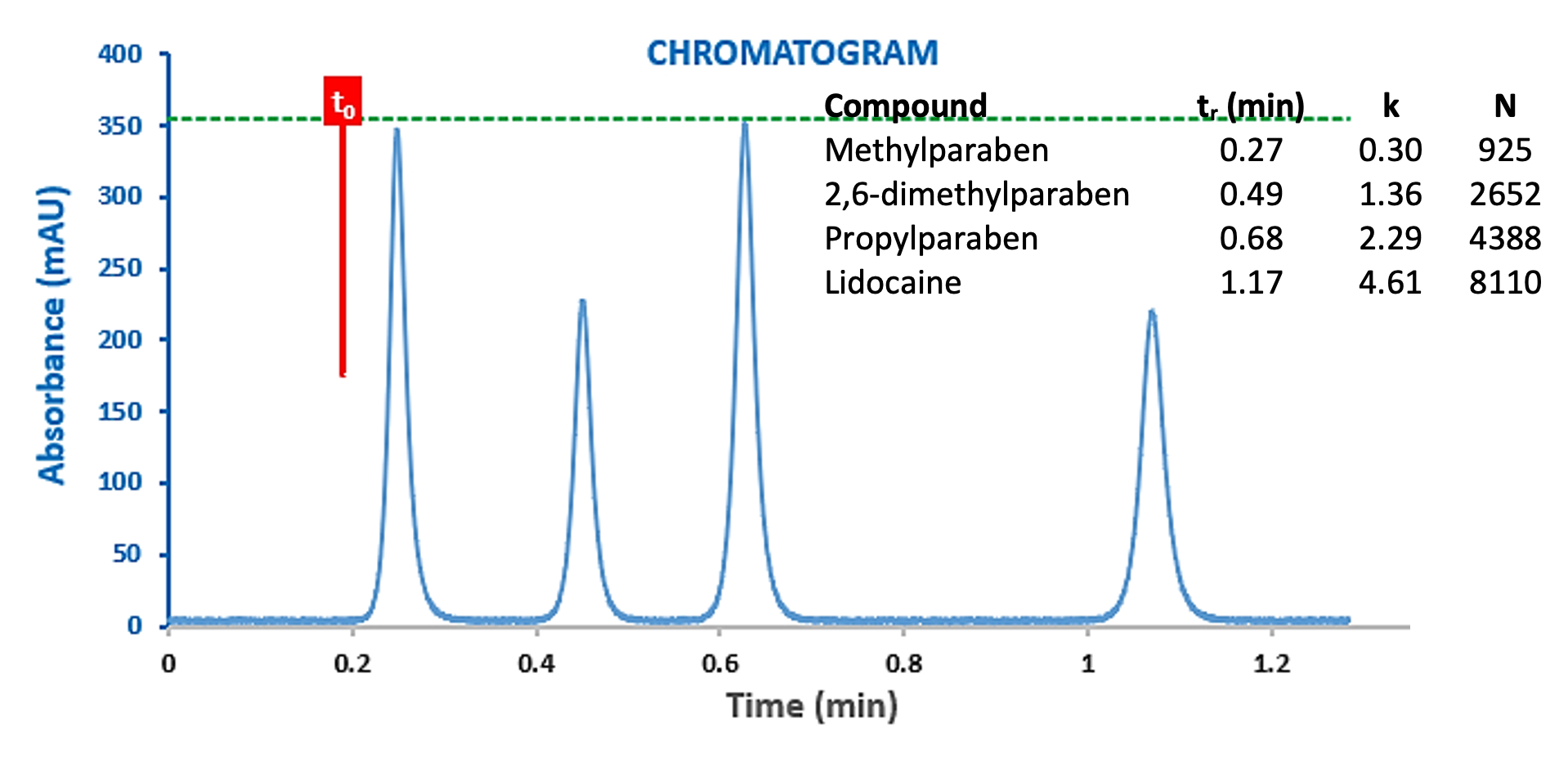
Figure 8: Chromatograms of a Lidocaine Preparation obtained with a ‘Standard’ UHPLC system with post-column flow splitting (1:2) at 0.5 mL/min (top) and 1.0 mL/min (bottom).
Whenever flow splitting is employed, one can mitigate some of the dispersion effects by keeping flow rates lower and ensuring the extra column volume and detector internal volumes are optimised. Note that my comment on detector internal volume is not to reduce the internal volumes as low as possible, as any reduction in flow cell volume, combined with the low sample volume post-split may significantly affect the sensitivity of the signal obtained. Again, for further information I would refer the reader to reference 2.
Detector data acquisition and processing parameters also play an important role in defining the peak dispersion in the chromatographic output. This is true not only for the sampling frequency (number of measurements per second) but also the data bunching rate (or time constant) which is often used as a smoothing function to reduce the level of noise within the signal. Figure 9 shows the same experiment as shown in Figure 8 (top), but with a higher time constant value.
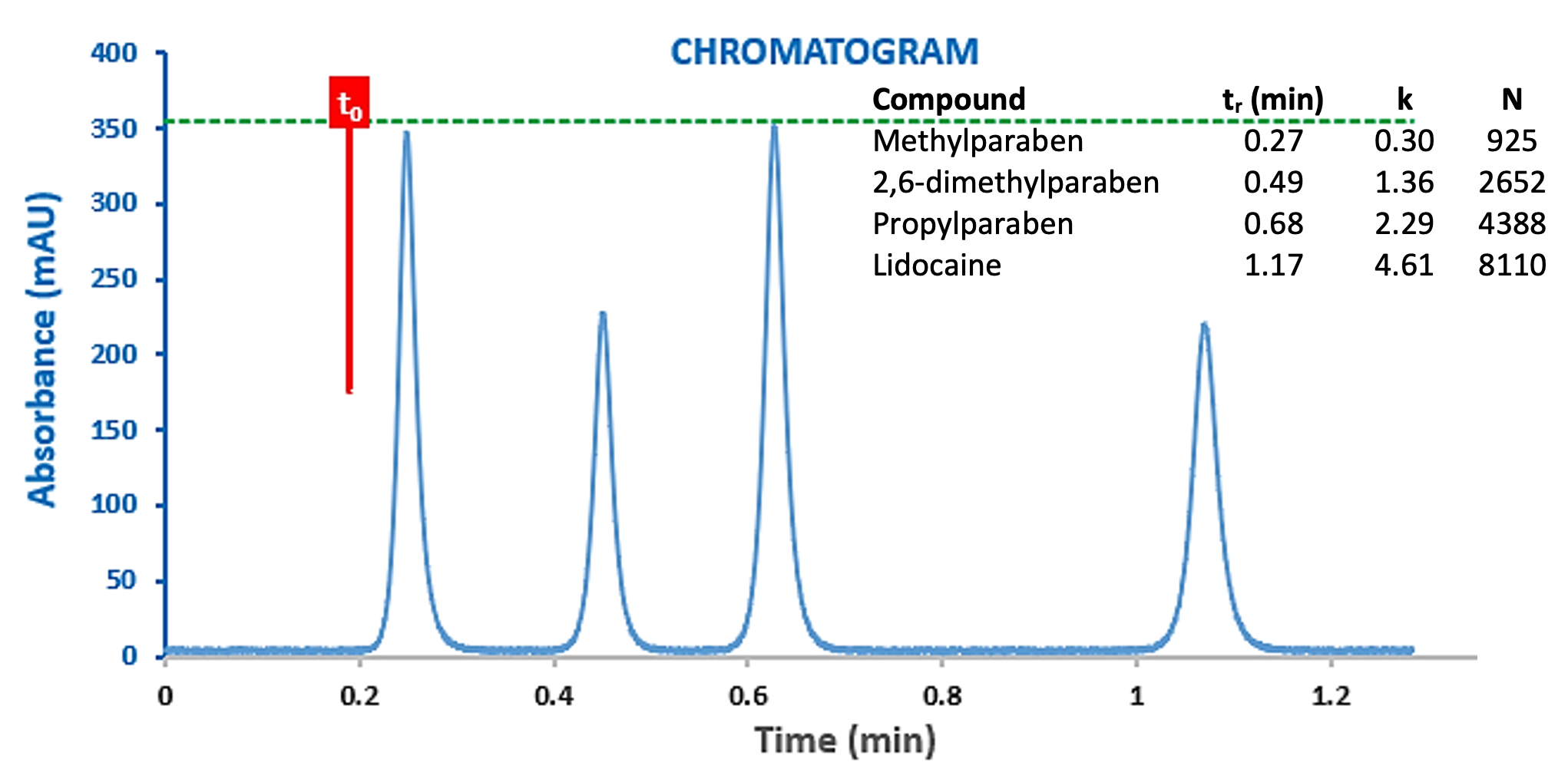
Figure 9: Chromatograms for the separation of a Lidocaine Preparation obtained with a time constant of 100ms (top) 1000ms (bottom).
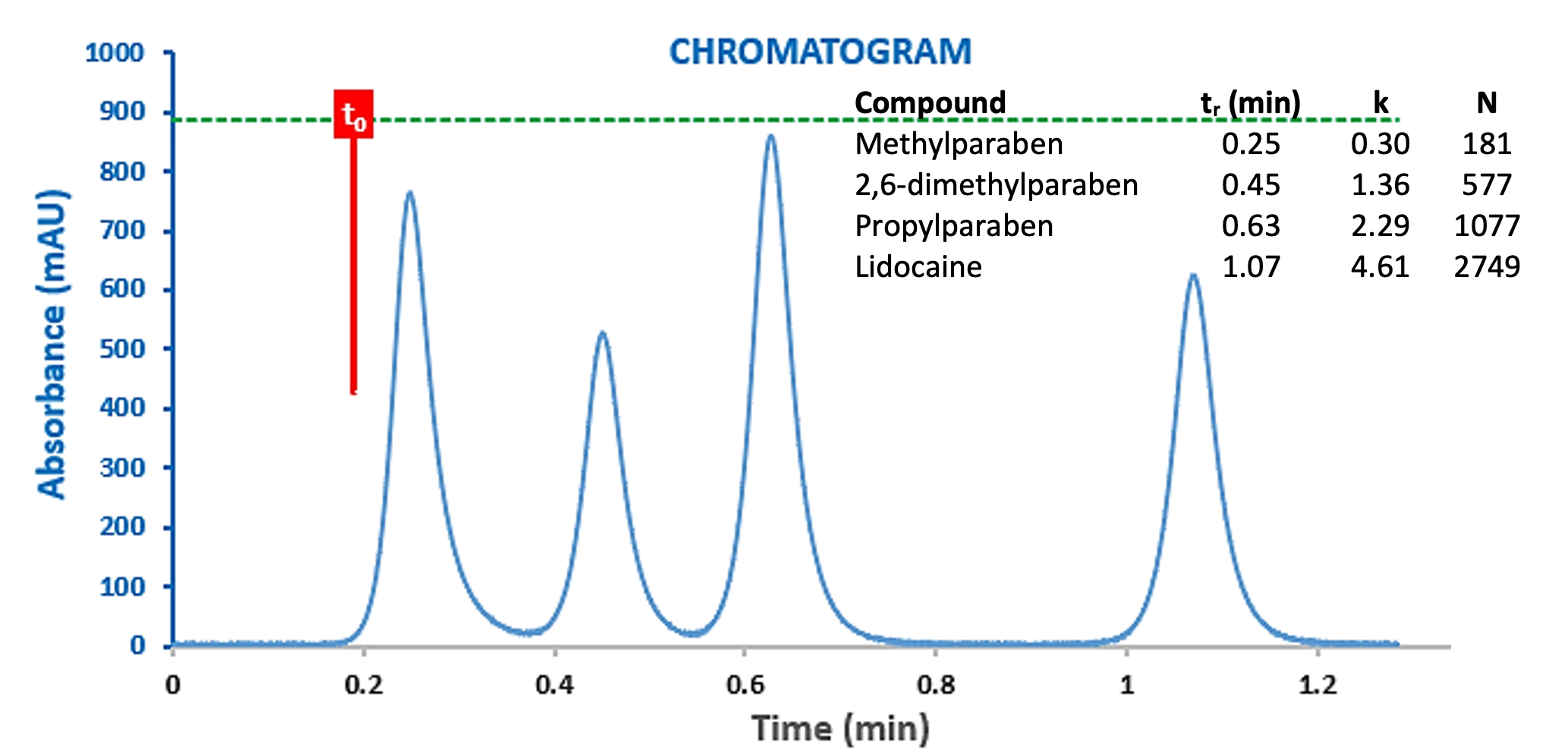
One can clearly see the effects of the higher data bunch/smoothing rate in the modelling of the chromatographic peaks, which has the same effect as adding a further, large, extra column volume into the UHPLC system. At this point the efficiency of the chromatographic peaks is very low and one can be fooled into thinking that there is a fundamental issue with the chromatographic hardware.One should pay particularly close attention to the optimisation of detector settings when using UHPLC for rapid separations.
In all cases above, we have concentrated on isocratic separations, as frankly, these separations highlight the issues of extra column broadening most starkly. In gradient analysis mode, the phenomenon of analyte ‘focussing’ at the head of the analytical column somewhat mitigates the dispersion effects of all components up to the head of the analytical column. Whilst gradient analysis may be seen as highly advantageous in reducing extra column effects, there are several other issues associated with dispersion in gradient HPLC which will form the basis of a future issue.
To summarise, we have used excellent modelling tools to investigate various dispersion effects on the efficiency of chromatographic peaks in a model application. It is hoped that the reader will have a deeper insight into some of the dominant factors which are important in determining peak dispersion and their typical values in UHPLC systems. We have also discussed various strategies to avoid unnecessary peak dispersion in terms of the UHPLC system components and detector hardware and data acquisition settings. There are many methodological factors which may also affect efficiency in UHPLC and these will be further explored in a future issue.
References
- https://www.crawfordscientific.com/chromatography-blog/post/putting-the-u-into-your-uhplc
- D.R.Stoll & K. Broeckhoven, LCGC North America 39(4), 159–166 (2021) (https://www.chromatographyonline.com/view/where-has-my-efficiency-gone-impacts-of-extracolumn-peak-broadening-on-performance-part-i-basic-concepts)
- D. Guillarme, B. Bobaly, & J. Veuthey LCGC North America 39(3), 144–145 (2021)(https://www.chromatographyonline.com/view/free-excel-software-liquid-chromatography)
- http://www.multidlc.org/dispersion_calculator
- L. R. Snyder, J. Chromatogr. Sci. 16, 223 (1978)
Investigating the Protective Effects of Frankincense Oil on Wound Healing with GC–MS
April 2nd 2025Frankincense essential oil is known for its anti-inflammatory, antioxidant, and therapeutic properties. A recent study investigated the protective effects of the oil in an excision wound model in rats, focusing on oxidative stress reduction, inflammatory cytokine modulation, and caspase-3 regulation; chemical composition of the oil was analyzed using gas chromatography–mass spectrometry (GC–MS).
Evaluating Natural Preservatives for Meat Products with Gas and Liquid Chromatography
April 1st 2025A study in Food Science & Nutrition evaluated the antioxidant and preservative effects of Epilobium angustifolium extract on beef burgers, finding that the extract influenced physicochemical properties, color stability, and lipid oxidation, with higher concentrations showing a prooxidant effect.
Rethinking Chromatography Workflows with AI and Machine Learning
April 1st 2025Interest in applying artificial intelligence (AI) and machine learning (ML) to chromatography is greater than ever. In this article, we discuss data-related barriers to accomplishing this goal and how rethinking chromatography data systems can overcome them.



