The LCGC Blog: Supercritical Fluid Chromatography: A Workhorse in Drug Discovery
The first report of using supercritical fluid for separations can be traced back to 1962. A publication by Klesper, Corwin, and Turner used dichlorodifluoromethane above its critical temperature to separate a mixture of metalloporphyrins (1). The authors defined this technique as “high-pressure gas chromatography,”with the term supercritical fluid chromatography (SFC) being introduced within the following decade. Interestingly, SFC predates the introduction of high-performance liquid chromatography (HPLC); however, for a long time, SFC failed to garner the popularity of HPLC. SFC was initially considered an extension of gas chromatography (GC) with supercritical carbon dioxide (though other supercritical fluids have been tried), which became the mobile phase of choice for several reasons, including low cost, low toxicity, and its environmentally friendly nature compared to other supercritical fluids. The addition of a polar alcohol (modifier), such as methanol, ethanol, or isopropanol, along with an acid or base in small proportions (additives) allowed SFC to gain popularity starting in the early 1980s due to the expanded range of analytes that could be eluted. When did the SFC revolution begin, one might ask? This can be traced back to the acetonitrile shortage in 2008 (2). A major shift towards improvements in SFC instrumentation was thusly observed, which in turn increased SFC use in a wide range of industries and applications. The introduction of novel back-pressure regulator design proved to be a game changer towards the robustness of SFC instrumentation and its eventual widespread adoption.
One specific area where SFC has gained traction is enantiomeric separations. Enantiomeric separations were typically done under normal-phase conditions, which consumed high amounts of organic solvents. Additionally, the lack of compatibility between mobile phase and mass spectrometry (MS) detection presented additional challenges. Furthermore, irreproducible retention times in normal-phase liquid chromatography (NPLC), owing to the presence of trace amounts of water, long equilibration times, and high viscosity resulting in high pressures, made the use of normal-phase separations challenging. On the other hand, SFC is compatible with MS while using lesser organic solvent and environmentally benign CO2 (3). This makes SFC a greener technique in comparison to NPLC. With a push towards more environmentally sustainable analytical methods, switching to SFC can be a step in the right direction. Additionally, with chiral separations being plagued by slow mass transfer kinetics, the low viscosity of the SFC mobile phase allows for higher flow rates with tolerable back pressure, reducing the contribution of mass transfer kinetics to band broadening or the C-term of the van Dimeter equation. Also, at high flow rates, there is less contribution from the B-term or the longitudinal diffusion term, which further makes SFC separation more efficient. A concomitant advantage of using high flow rates is the much faster analysis of samples, which is of utmost importance in the modern day. With the understanding of its advantages, we can now analyze why SFC is fundamental to the small molecule drug discovery process.
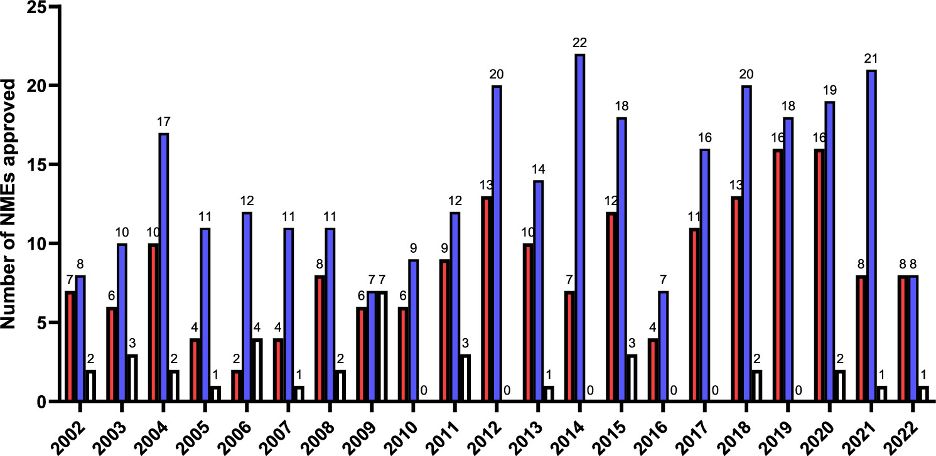
On a global scale, small molecule drugs account for 90% of all drugs marketed on a yearly basis (4). A significant number of these small molecule drugs approved by the FDA in the last 20 years have one or more chiral centers (or chiral planes). As can be seen in Figure 1, which compares the number of achiral, single-enantiomer, and racemic drugs approved by the FDA from 2002 to 2022, the number of single-enantiomer drugs approved is significantly higher than racemic drugs. This stems from the fact that generally, one of the enantiomers is biologically active, whereas the other is less active, or in some cases, toxic.
Figure 1: Comparison of the number of achiral (red), single enantiomer (blue), and racemic (white) small molecule NMEs approved by the FDA between 2002 and 2022. Reprinted with permission from American Chemical Society (Copyright © 2024). (5)
An account which has probably been told at the beginning of every undergraduate organic chemistry class discussing chirality is the story of the drug thalidomide. While (R)-thalidomide successfully treated morning sickness in pregnant women, the (S)-enantiomer caused embryo-toxic and teratogenic effects. However, this is not the only example of the detrimental effects of one enantiomer; other examples include the d-isomer of Levodopa, which causes granulocytopenia, and the d-isomer of carnitine, which causes symptoms of myasthenia gravis, to name a few. This led to the momentous 1992 FDA guideline which required the testing of individual enantiomers in in vivo samples early in drug development. In the early drug discovery phase, thousands of compounds are synthesized and assessed. At this step, SFC instrumentation plays a pivotal role in enabling high-throughput drug discovery. Samples for purification are screened with analytical SFC instrumentation with a wide variety of chiral stationary phases to obtain baseline separation of enantiomers. Fortunately, most chiral stationary phases that work with either normal-phase or reversed-phase HPLC also show enantio-recognition under SFC conditions. After a method is obtained, it is then transferred to a preparative instrument to isolate the individual enantiomers. The isolated fractions are then dried down before being sent out for assays. One operational advantage of using SFC resides in the fact that the mobile phase consists of carbon dioxide and a low boiling polar alcohol that can be easily removed using a rotovap. This decreases the turnaround time, which is a major factor to consider, given the time-sensitive nature of the drug discovery process.
Apart from chiral separations, SFC is being utilized in achiral analysis and purifications as well. Several meticulously designed stationary phases for SFC have been introduced by different vendors, such as 2-ethylpyridine, diol, and DEAP (diethylamino), just to name a few (3). These stationary phases have paved the way for SFC in chromatographic analysis of achiral compounds.
However, SFC, despite its advantages, does not replace HPLC analysis. The adoption of SFC in later stages of the drug development process is painfully slow due to the issues with run-to-run reproducibility and the high cost of instrumentation (6). Another limitation of SFC is the narrow range of analyte polarity that can be analyzed. With the focus of drug development expanding to hybrid modalities, such as peptides, RNAs, and other biologics, from an analytical standpoint, SFC is severely lacking compared to HPLC. With that being said, small molecules still dominate the drug landscape, and SFC plays a vital role in enabling small-molecule drug discovery.
Lastly, we would like to dedicate this blog to Dr. Terry Berger, also known as the father of modern supercritical fluid chromatography, who recently died. His work on SFC instrumentation, including developing its applications, has led to widespread adoption of the technique in both academia and industry. As Isaac Newton once said, “If I have seen further, it is by standing on the shoulders of giants,”and today, for analytical scientists involved in the SFC development, most of it stands on the groundwork laid by Dr. Terry Berger.
References
(1) Klesper, K. High Pressure Gas Chromatography Above Critical Temperatures, J. Org. Cher 1962, 27, 700–701.
(2) Veuthey, J. -L.The Revival of Supercritical Fluid Chromatography in Pharmaceutical Analysis. LCGC Europe 2017, 30 (11), 609–610.
(3) Majors, R.; Berger, B.; Berger, T. A Review of Column Developments for Supercritical Fluid Chromatography. LCGC North America 2010, 28 (5), 344–357.
(4) Makurvet, F. D.; Biologics vs. Small Molecules: Drug Costs and Patient Access, Medicine in Drug Discovery 2021, 9, 100075. DOI: 10.1016/j.medidd.2020.100075
(5) McVicker, R. U.; O’Boyle, N. M. Chirality of New Drug Approvals (2013–2022): Trends and Perspectives. J. Med. Chem. 2024, 67 (4), 2305–2320. DOI: 10.1021/acs.jmedchem.3c02239
(6) Tarafder, A.; Collier, S. M.; Hill, J. F. 3. Addressing Robust Operation and Reproducibility in SFC, in: R. Gérard (Ed.),Supercritical Fluid Chromatography: Volume 1, edited by Gérard Rossé, Berlin, Boston: De Gruyter, 2019, 73–94. DOI: 10.1515/9783110500776-003
About the Authors
Diapayan Roy is a senior scientist in the synthetics separations team at Amgen. His work focuses on chiral and achiral method development and purifications. He completed his PhD in 2020 under supervision of Dr. Daniel Armstrong at University of Texas at Arlington. His graduate research was focused on chiral separations with HPLC and SFC.

Muhammad Qamar Farooq is a scientist in the synthetics separations team at Amgen. His work focuses on analysis and purification of small and hybrid molecules using chromatographic techniques. He completed his PhD in 2022 under supervision of Dr. Jared Anderson at Iowa State University. His graduate research was focused on utilization of ionic liquids and deep eutectic solvents for analytical separations. He is also an executive committee member of ACS SCSC (Subdivision of Chromatography & Separations Chemistry).


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



