The LCGC Blog: Optimizing Gas Chromatography Using Column Dimensions
This article explores how stationary‑phase chemistry and column temperature programmes can impact GC separations, navigating various choices to maximize resolutions.
FR Design/stock.adobe.com

Optimizing gas chromatography (GC) separations typically involves making some informed choices around stationary‑phase chemistry and column temperature programs.
Often, phase choice will be influenced by separations gleaned from literature, while temperature programming tends to be more of an iterative process involving a good deal of trial and error.
However, some judicious initial choices of GC column dimensions, and even a change of column dimension during method development, can lead to significant improvements in resolution. This tends to be a much under-used method development tool, perhaps understandably, as changing columns can be both costly and, especially when using MS detection, time consuming.
Nonetheless, some knowledge of the relationships between resolution and column length, internal diameter and film thickness can lead to the best column choice to begin method development or to get you out of trouble when your stationary phase chemistry choice or temperature program optimization do not produce the required results!
The primary relationships between resolution and column dimensions are outlined in equation 1 and Table 1:
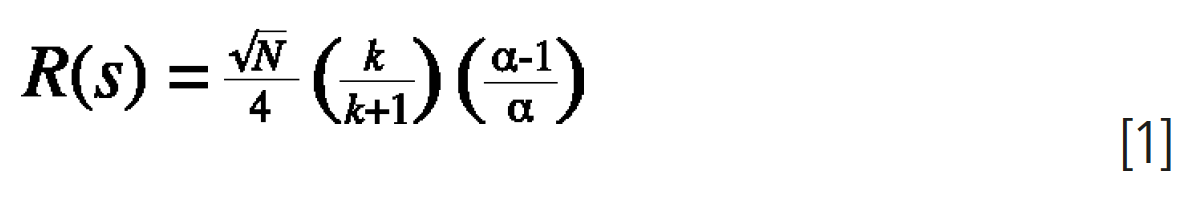
- Efficiency (N) is a function of: carrier gas type, column length (L), column internal diameter (dc)
- Retention Factor (k) is a function of: temperature, film thickness (df), column internal diameter (dc)
- Selectivity (a) is a function of: temperature (T), stationary phase chemistry
So how can we relate the various GC column dimensions to resolution (Rs)? Combining equation 1 with the information in Table 1 we can summarise the overall effects of column dimensions on chromatographic resolution using the following general relationships and caveats:
Column Length (L)
- Doubling column length doubles efficiency (N) which improves resolution by a factor
of 1.4 - Doubles analysis time (or by approximately 1.5–1.75 times for gradient temperature programming)
- Increases column costs
- Need to consider the effects of elution temperature with increasing column length–may need to adjust oven temperature program
Column Internal Diameter (dc)
- Affects efficiency, retention, carrier flow rate, capacity and pressure drop across the column
- Column internal diameter is inversely proportional to column efficiency – halve column diameter, double efficiency, increase resolution by factor of 1.4
- Column head pressure is an inverse square function of column radius–so halving column diameter will increase head pressure (for an equivalent linear velocity) by a factor of 4
- Column capacity increases with column internal diameter, however capacity is also dependant on the stationary-phase type, film thickness, and the nature of the analytes
Stationary-Phase Film Thickness (df)
- Affects retention, inertness, capacity, resolution, and bleed
- Film thickness is directly proportional to retention time (or a ratio of 1.5:1 for gradient temperature programming)
- Thick stationary-phase films give retention for highly volatile analytes
- Increasing film thickness allows retention of volatile analytes at temperatures at or above ambient
- Doubling stationary phase film thickness gives an increase of around 20 °C in elution temperature
- Retention of late eluting (high boiling point) analytes is reduced using thinner film columns
- Early eluting analytes (k<2) are better resolved using thicker film columns
- Resolution may DECREASE for analytes with retention factor (k) values greater than around 5 with INCREASING film thickness
- Thicker films bleed more–upper temperature limits of thick film columns will be lower
- Thicker film columns are more inert as the film shields the analyte from active sites on the silica tubing (therefore, better peak shape for polar and active compounds)
- Thicker film columns have higher analyte capacity, and so may reduce peak fronting
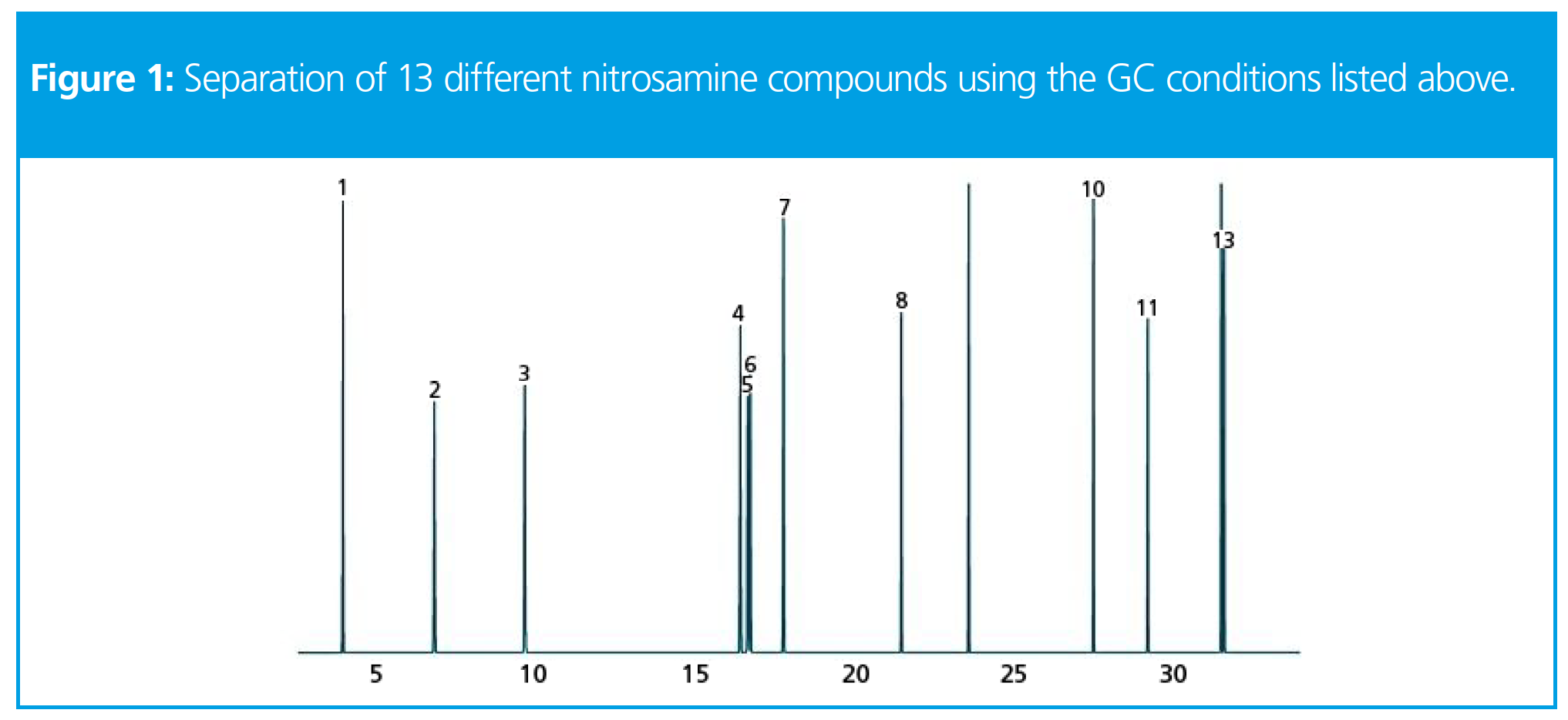
As always, it is better to visualize these effects using real-life examples, and so here we walk through some of the effects of changing GC column dimensions with the separation of nitrosamine standards.
GC Conditions
Column: 5% phenyl polydimethylsiloxane (ultra-deactivated), 30.00 m, 0.25 mm ID, 0.25 µm
Carrier Gas: Helium, Constant Flow @ 1.59 mL/min
Average Velocity: 35.00 cm/s
Outlet Pressure: 14.70 psi (atmospheric pressure)
Oven Temp: 40 °C (hold 5 min) to 80 °C @ 4 °C/min to 245 °C @ 8 °C/min
Analytes
1. N-Nitrosodimethylamine, 2. N-Nitrosomethylethylamine, 3. N-Nitrosodiethylamine, 4. N-Nitrosopyrrolidine, 5. N-Nitrosomorpholine, 6. N-Nitrosodipropylamine, 7. N-Nitrosopiperidine, 8. N-Nitrosodibutylamine, 9. N-Nitrosodiethanolamine, 10. N-Nitrosodiphenylamine, 11. N-Nitrosonornicotine, 12. N-Nitrosodibenzylamine, 13. N-Nitrosonornicotine ketone
From this separation we are particularly interested in optimizing the separation of compounds 4 through 7, and will use the relationships described above to obtain an optimized separation for just these compounds of interest.
Figure 2 shows an expansion of the chromatogram to help us visualize the separation more clearly. In each of the separation optimizations, as we change the column dimensions, we will maintain a constant carrier gas linear velocity by adjusting the column head pressure to obtain 35cm/s for the carrier gas (helium), unless otherwise stated.
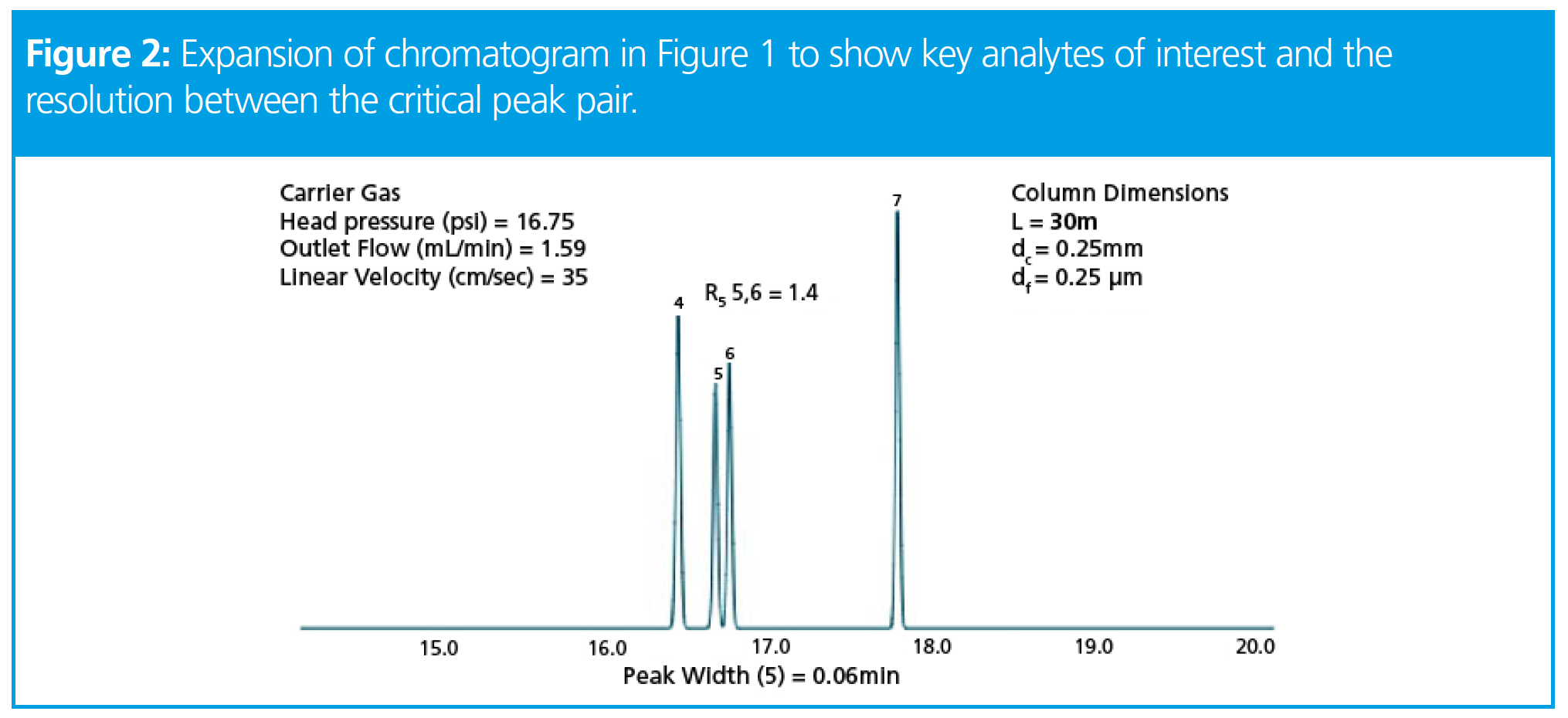
At a resolution of 1.4, this method is going to be difficult to implement in practice and therefore we need to optimize the separation.Let’s see what happens when we change the column length to 15 and 60 meters to assess the effects.
Ouch! It would seem that as we increase column length, the resolution increase which was promised above, has not been realized! We can see that indeed the 15 m column produces less efficiency (wider peak width at a constant linear velocity) and that the longer column delivers higher efficiency, again obvious from the decreased peak width when compared to the 30 m column. So what has gone wrong?
Well, the eagle-eyed among you will have noted the caveat that while longer columns deliver higher efficiency, we may also need to consider the oven temperature profile for this to offer improved resolution. If we were to calculate the actual elution temperature (for example, the oven temperature at which the peak elutes from the GC column) for peak 5, for example, the results would be:
15 m column = 71 °C
30 m column = 94 °C
60 m column = 122 °C
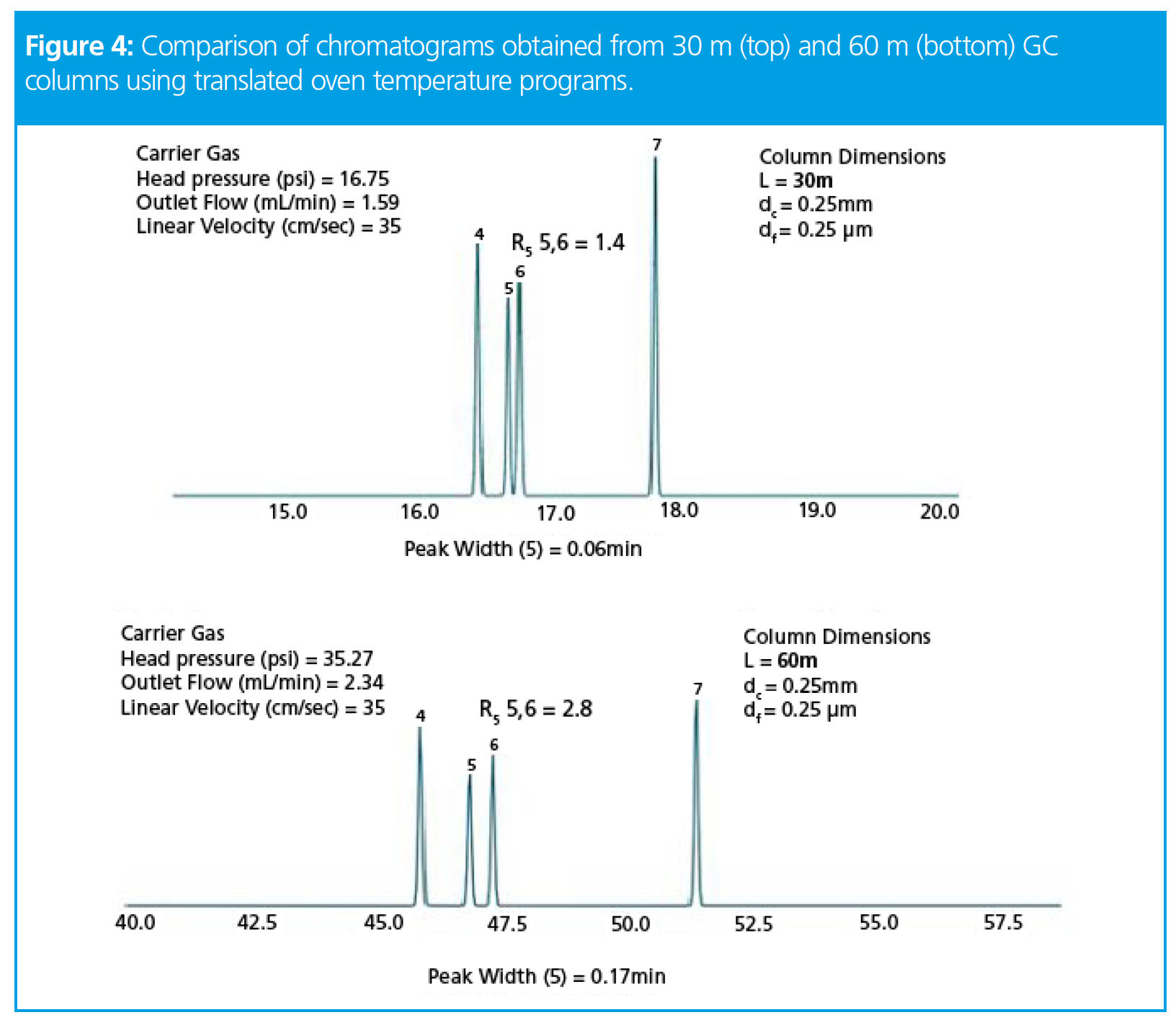
We know from equation 1 and the accompanying notes that selectivity is influenced by changing column temperature, and so in order to optimize resolution when changing column length, we may also need to optimize the oven temperature program. The most practical way to do this is to use a program which enables translation of the oven temperature program (offered by most of the vendors of GC equipment), the results of which are shown in Figure 4.
GC Conditions
Carrier Gas: Helium, Constant Flow @ 1.59 mL/min
Average Velocity: 35.00 cm/sec (30m)
Outlet Pressure: 14.70 psi (atmospheric pressure)
Oven Temp: 40 °C (hold 5 min) to 80 °C @ 4 °C/min to 245 °C @ 8 °C/min (30 m column)
40 °C (hold 17 min) to 80 °C @ 1.2 °C/min to 245 °C @ 2.4 °C/min (60 m column)
It will be obvious from Figure 4 that by translating the oven temperature program we have managed to significantly improve the resolution between the critical peak pair. It is certainly something we could comfortably work with on a routine basis. However, you will also have noticed that, alongside the new oven temperature program, we now have a much longer run time and that the chromatographic efficiency has significantly decreased (wider peaks than obtained with the original temperature program). However, for the huge increase in resolution we can live with the reduced efficiency and I’m sure the analysis time could have been shortened with a little experimentation.
Now let’s turn our attention to both column internal diameter and the effects of changing column such as on resolution. Figure 5 shows the results using a 30 m column and internal diameters of 250 (original), 180 and 530 mm. The original oven temperature program of Figure 1 was used in each case.
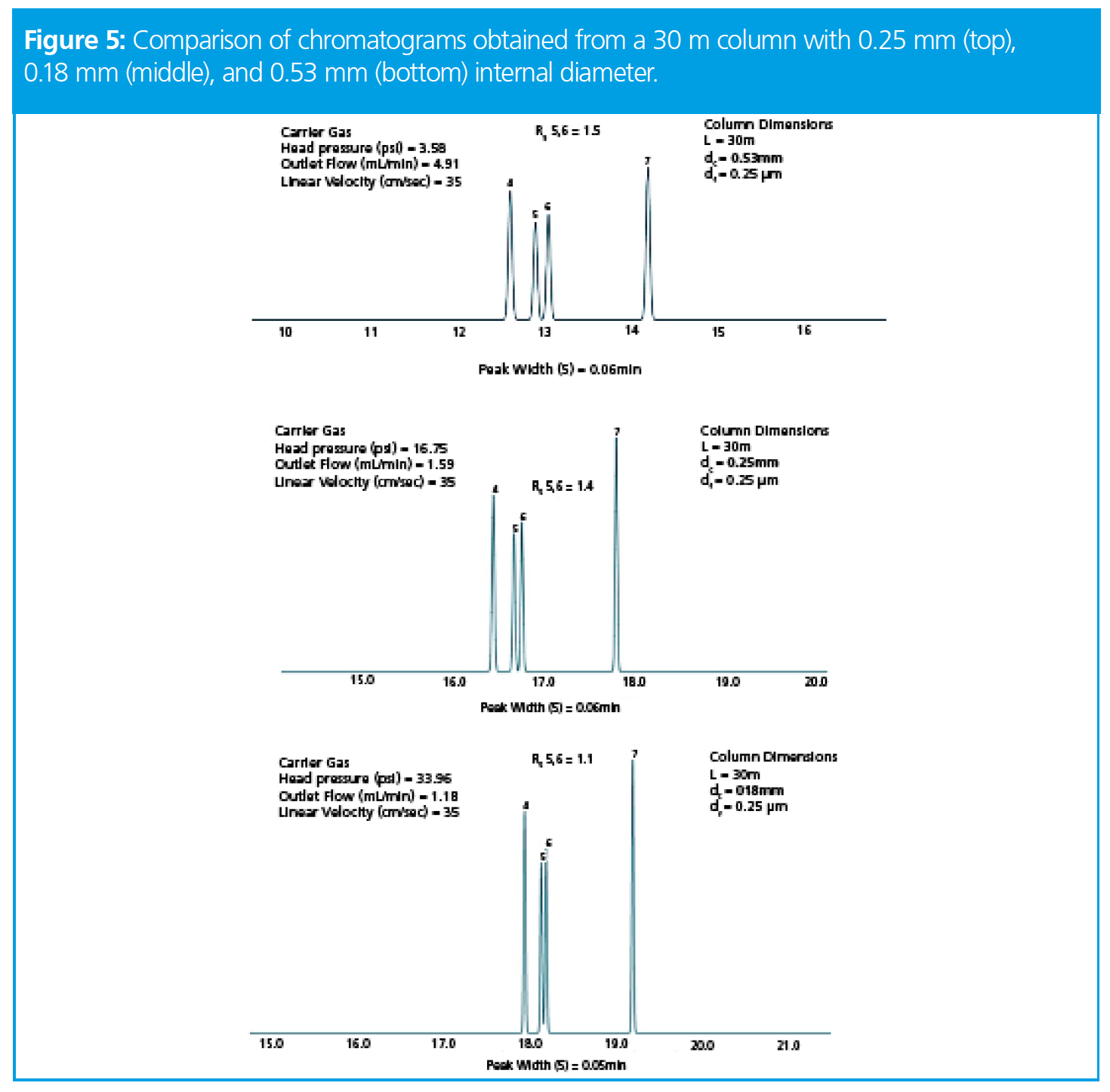
From Figure 5, we can see that we lose some resolution when the internal diameter is reduced and gain a little when it is increased (and the opposite effect is seen with efficiency or peak width). This makes sense of course, given that the k term increases when the internal dimeter is reduced, acting to reduce resolution and in this case we assume that the increase in k is greater than the increase in efficiency (N), thus resolution decreases with reducing internal diameter. Later we will look at a special case where when can overcome this.
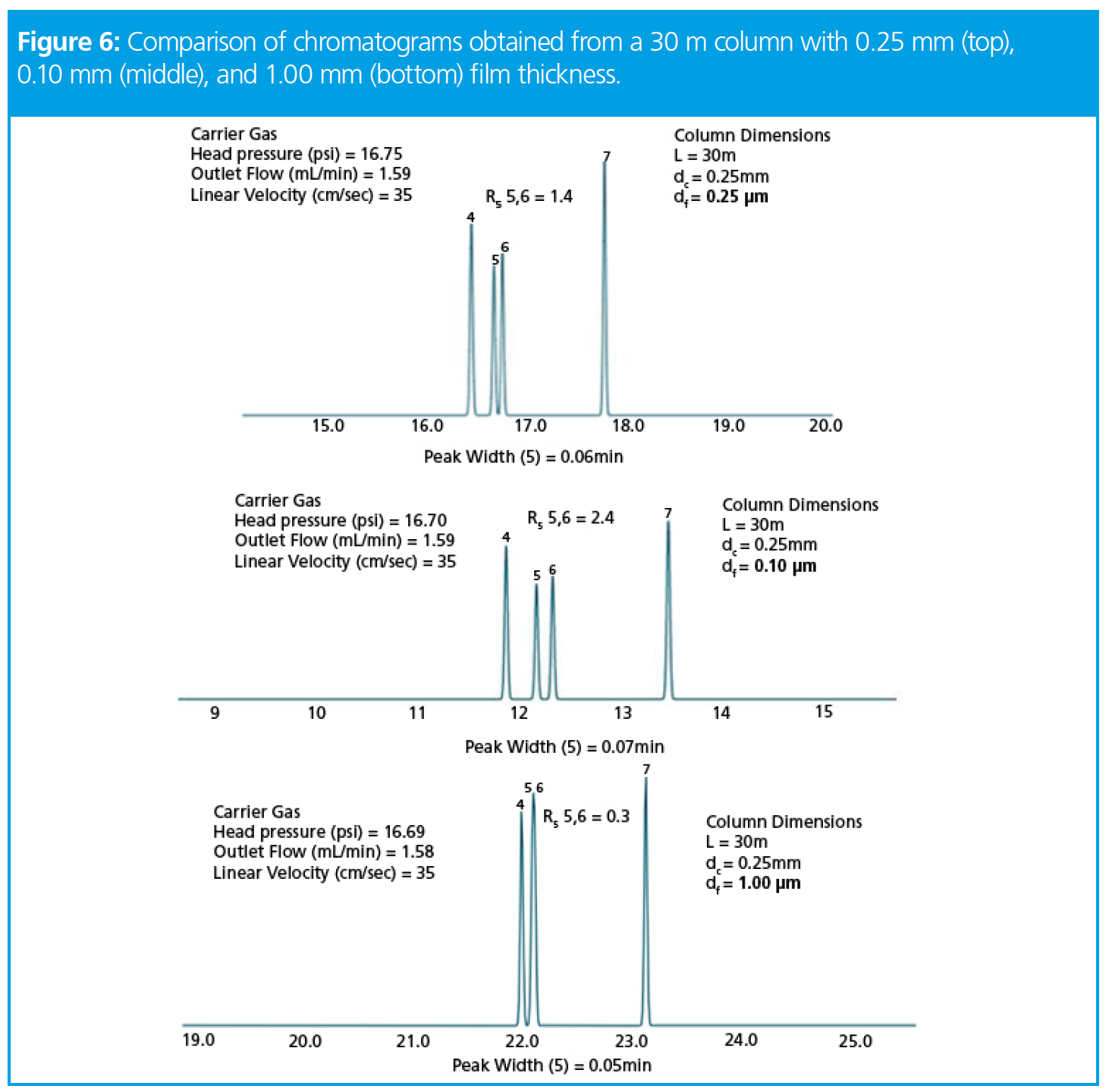
From Figure 6, we can see that again the data follows our prediction and as we increase film thickness, retention factor increases and resolution decreases. What we also need to consider is that the column loadability (the amount of analyte we can load onto the column before peak shape begins to deform), is reduced by a factor of four when we move to the thinner film and increases by a factor of approximately 5 when we move to the thicker film. So, when making our stationary-phase film thickness choice, we must always consider the column (stationary phase) loadability as well as the volatility of the analytes, and that more volatile analytes require thicker films for good retention.
To end, there is one reasonably special case that we should consider, and that is the combination of smaller internal diameter columns with relatively thin films. Here the effects of the increased retention are counterbalanced by the reduction in film thickness, and we can often obtain very satisfactory separations. The added advantage is that to obtain a reasonable flow rate, column linear velocities are typically higher and therefore analysis tends to happen in shorter timeframes. This combination of smaller internal diameter with thinner films is often coined “fast GC” as, not only are column flow rates higher, but shorter columns can often be used without any meaningful reduction of resolution.
Figure 7 shows an example of this in action. Note that the linear velocity has been optimised and the temperature program translated.
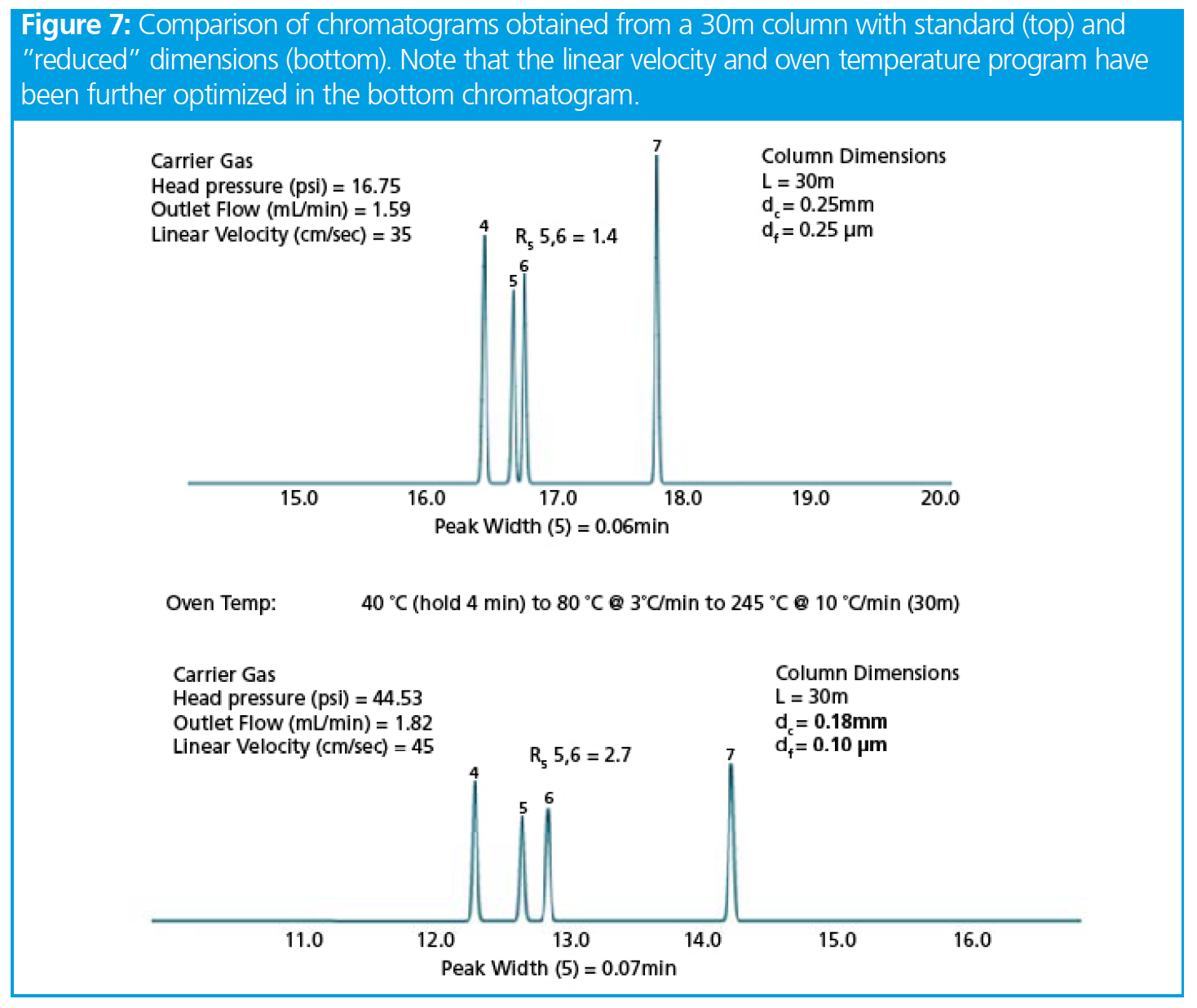
From Figure 7 it should be immediately obvious that we are approaching the resolution obtained with the 60 m column (Rs = 2.8), yet we have obtained this resolution in quarter of the analysis time hence the power of what has become known as “Fast GC.” This would be a very acceptable set of conditions for routine analysis, provided that we do not load too much sample onto the column, which of course can be controlled by altering the inlet split ratio. We have explored the basic relationships between column dimensions and resolution, considering the many caveats and rules of thumb which might guide our choices during method optimization. While it is not highly convenient to alter columns during the method development process, sometimes, if we run out of resolving power and cannot obtain a satisfactory separation by altering the oven temperature program, we have no choice but to alter either the stationary-phase chemistry (and effectively start all over again!) or we can make some smart choices in altering the column dimensions to squeeze every last drop of resolution from our chromatographic system.
In writing this article I used some very useful tools which can help to model the separations that we may obtain and the translation of oven temperature and column flow conditions. I’ve listed links to these tools below:
Pro EZGC Chromatogram Modeler from Restek (Bellefonte, Pennsylvania, USA): www.restek.com/proezgc
Agilent (Santa Clara, California, USA) GC Method Translator: www.agilent.com/en/support/gas-chromatography/gcmethodtranslation
Agilent (Santa Clara, California, USA) GC Calculators: www.agilent.com/en/support/gas-chromatography/gccalculators
Hopefully, you will carefully consider your choice of column dimension when planning and optimizing your next separation!
Tony Taylor is the Chief Scientific Officer of Arch Sciences Group and the Technical Director of CHROMacademy. His background is in pharmaceutical R&D and polymer chemistry, but he has spent the past 20 years in training and consulting. He has trained and consulted with thousands of analytical chemists globally and is passionate about professional development in separation science, developing CHROMacademy as a means to provide high-quality online education to analytical chemists.

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.











