*** TEST *** Estimation of the Main Sources of Uncertainty in Chromatographic Analysis: Determination of Biogenic Amines ***TEST ***
LCGC North America
This article establishes the estimation of the uncertainty associated with the chromatographic determination of biogenic amines. The authors identify and estimate each source of uncertainty to establish the accuracy of results and to obtain a better understanding of the method. Thus, measurement uncertainty was split into two sections: uncertainty related to the working conditions, which considers the equipment used, and inherent uncertainty, which includes the chemical stages indicated in the procedure as well as calibration sources, taking into account the existence of the matrix effect. Recovery studies also were made to quantify the contribution of bias to the overall uncertainty. This parameter was calculated for the determination of biogenic amines in different types of samples.
In recent years, the subject of the evaluation of measurement uncertainty in analytical chemistry has generated a significant level of interest and discussion. The International Organization for Standardization (ISO) has published a guide for the harmonization of the expression of results with uncertainty (1). This guide has been adapted to the chemical problem by Eurachem, a network of European organizations, and the Cooperative on International Traceability in Analytical Chemistry (CITAC) (2). Three approaches are proposed: bottom-up, top-down, and in-house validation. The first approach involves the exhaustive division of the analytical method into its steps and the identification, quantification, and combination of all sources of uncertainty. The second approach includes the treatment of interlaboratory information, and the third approach involves the use of information obtained from repeated analyses derived from in-house validation of analytical methods.

In some cases, the very high number of combinations of commodities and analytes, along with the absence of certified reference materials, represents a challenge for any of the approaches indicated by Eurachem (3), and occasionally, a combination of the bottom-up approach and the data derived from in-house validation (4) must be used.
Although the Eurachem guide shows some examples, analytical chemists frequently have regarded the estimation of uncertainty as too theoretical and not suitable for the estimation of uncertainties of complex chemical techniques. This is because identifying and quantifying all sources of uncertainty is difficult and laborious, for procedures consisting of numerous steps, many of which are not always clearly distinguishable. Analytical chromatographic procedures can be good examples of this situation, in which sources of uncertainty exist (5,6) such as sample handling, derivatization reaction, calibration function, matrix effect, and separation.
Among the three approaches mentioned earlier for routine analysis, the combination of the bottom-up approach and the in-house validation data shows the most available information. Nevertheless, neither identification nor quantification of the uncertainty associated with the chemical factors of the method has been combined, so two sources related to the factors not controlled by the operator could be introduced. The first depends upon the chemical stages indicated in the procedure, and the second is associated with the chemical calibration process.
This paper describes a general procedure for evaluating the uncertainty of the analytical results obtained in the determination of biogenic amines in solid and liquid samples using a high performance liquid chromatography (HPLC) method after derivatization with dabsyl chloride (7,8). Bearing in mind that all of the analytes are not always present in the samples analyzed, the bias has been evaluated using recovery studies.
The definition and evaluation of the new chemical uncertainty sources, as well as the uncertainties checked by recovery factors, have been used to estimate the overall uncertainty. This new approach can be considered a reliable way to establish the accuracy of the results and to obtain a better understanding of the method.
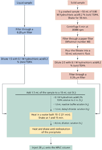
Figure 1: Procedure for the chromatographic determination of biogenic amines in solid and liquid samples.
Experimental
Apparatus:
The HPLC system consisted of a Hewlett Packard 1050 series liquid chromatograph equipped with a variable-wavelength UV-vis detector, a model 3396-A integrator (all from Agilent Technologies, Palo Alto, California), and a 7125 loop injector with a 20-mL sample loop (Rheodyne, Inc., Cotati, California). A 244 mm 3 4.4 mm, 5-mm dp Lichrospher (Merck, KGaA, Darmstadt, Germany) 100 RP-18 column linked to a 10 mm 3 4.6 mm Lichrospher guard column was used for all separations.
A Heidolph (Schwabach, Germany) Reax 2000 vortex mixer, a Precisterm (J.P. Selecta s.a., Barcelona, Spain) s-137 thermostated bath, and a BHG Fixette 2 (Hermle Labortechnik GmbH, Wehingen, Germany) centrifuge also were used. All pH measurements were made with a Crison (Madrid, Spain) 2000 pH meter equipped with a combined AgCl-glass electrode assembly.
Chemicals and reagents: Amine standard solutions:All amine standards were purchased as hydrochloride salts of the highest purity available. Tryptamine (TRY), phenylethylamine (PHE), spermine (SP), and spermidine (SPD) were obtained from Fluka (Neu-Ulm, Germany); histamine (HT), cadaverine (CAD), putrescine (PUT), and tyramine (TYR) were obtained from Sigma (St. Louis, Missouri), and 1,7-diaminoheptane (as internal standard) was obtained from Aldrich (Steinheim, Germany). Stock solutions of the biogenic amines containing 1.0 g/L were prepared by dissolving them in 0.1 mol/L hydrochloric acid containing 0.2% (w/v) 3,39-thiodipropionic acid (TDPA) as an antioxidant. They were kept frozen at 220 °C.
Solutions for dabsylation reaction: The 12.4 mmol/L dabsyl chloride solution was prepared by dissolving 40 mg of dabsyl chloride (Fluka) in 10 mL of acetone, (Merck, Darmstadt, Germany), followed by ultrasonic treatment for 15 min and filtering through an Anotop (Whatman, Kent, United Kingdom) filter (Anopore inorganic membrane). The filter had a pore size of 0.20 mm and a diameter of 10 mm. The solution was filtered into a brown glass vial and stored at 220 °C. The reaction buffer medium consisted of 1.06 g of sodium carbonate (Panreac, Barcelona, Spain) in 50 mL of water. The dilution solution was a mixture of 50 mL of acetonitrile (Panreac), 25 mL of ethanol (Panreac), and 25 mL of eluent A (see Chromatographic Solutions).
Chromatographic solutions: Eluent A, consisting of 40 mmol/L sodium acetate (Panreac), 10% (v/v) dimethylformamide (Fluka), and 0.23% (v/v) triethylamine (Carlo Erba, Milan, Italy), was adjusted to pH 5.0 with diluted acetic acid (Panreac). Eluent B consisted of 87.5% acetonitrile (Panreac), 10% tert-butylmethyl ether (Fluka), and 2.5 % (v/v/v) water.
All glassware was rinsed thoroughly with 70% ethanol and water and dried before use. Glass vials for standards were heated at 500 °C for 3 h to remove any organic contaminants. Highly purified water (Milli-Q, Millipore, Bedford, Massachusetts) was used throughout for the preparation of buffers and reagents.
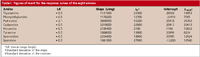
Table I: Figures of merit for the response curves of the eight amines
Procedure
Sample preparation:
Liquid samples (beers and wines) were filtered through a 0.20-mm membrane Millipore filter and diluted as indicated in Figure 1.
Solid samples (malt and maize) were ground and homogenized, and approximately 5 g of the sample was accurately weighed. Next, 50 mL of 0.80 mol/L hydrochloric acid, containing 0.2% (w/v) TDPA solution, were added and shaken for 18 min. The mixture was centrifuged for 5 min at 3000 rpm, removing the solid phase by filtration through filter paper (Whatman, n° 40). The filtrate was poured into a 50-mL volumetric flask and diluted to volume with 0.8 mol/L hydrochloric acid with 0.2% (w/v) TDPA. This extract was filtered again through a 0.20-mm membrane and diluted as indicated in Figure 1.
Dabsylation reaction: An aliquot of 1.5 mL of diluted beer or hydrochloric acid extract was transferred to a vial, and 0.5 mL of 0.1 mol/L hydrochloric acid solution with 0.2% (w/v) TDPA was added. Then it was adjusted to approximately pH 8.2 with 1.8 mL of reaction-buffer solution. After thorough mixing on a vortex-mixer, 1.6 mL of dabsyl chloride solution was added, and it was mixed again. The mixture was heated in a water bath for 21 min at 70 °C, and shaken at 1 and 15 min. Then, 4.6 mL of the dilution solution was added and allowed to stand for approximately 20 min in the water bath, and was shaken from time to time.
Chromatographic analysis: The C18 column was equilibrated at 40 °C with a mobile phase consisting of 45% eluent A and 55% eluent B. An aliquot of 20 mL of the dabsyl derivative solution was injected and eluted at a flow rate of 1.0 mL/min using the following gradient profile: 55% eluent B for 3 min, 75% B for 13 min, 100% B for 10 min, 100% B for 10 min, back to 55% B for 5 min, and finally, 55% B for 15 min. The detection wavelength was 446 nm. Figure 2 shows a typical chromatogram of the dabsylated amines.
Quantitative analysis: The data for an external calibration were collected for five concentrations (ranging from 0.030 to 0.500 mg/L and as high as 2 mg/L for putrescine) of biogenic amine standards using triplicate responses at each concentration and randomized arrangement. The type of analytical response (that is, peak height or peak area) as well as the convenience of an internal standard have been detailed in previous papers (8,9). The results showed that the best analytical response was peak area, and the use of an internal standard (1,7-diaminoheptane) was not necessary for quantitative analysis, although it was useful for qualitative purposes (9). Table I shows the figures of merit for each analyte.
Theoretical Aspects of the Estimation of Uncertainty
The estimation of uncertainty is simple in principle and involves the following steps:
Specify the measurand: This step includes a description of the mathematical relationship between the measurand and the parameters upon which it depends. These include constants, measured quantities, and calibration standard values. Furthermore, the procedure of the analytical method should be written.
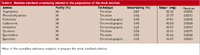
Table II: Relative standard uncertainty related to the preparation of the stock solution
Identify uncertainty sources: In this step, all the main uncertainty sources for each of the parameters that affect the value of the measurand should be identified. Instrument effects, reagent purity, measurement conditions, and sample handling are typical sources of uncertainty that could be termed operational or working uncertainty because they arise from physical operations carried out by the operator.
However, for an analytical method in general and for a chromatographic method in particular, we should estimate the uncertainty associated with those chemical stages indicated in the procedure that are not controlled by the operator because of their dependence on various chemical factors. These include the efficiency of the extraction process or the yield of the derivatization reaction used to improve the detection of the analytes. In our opinion, these types of uncertainties should be included under the denomination of intrinsic uncertainty.
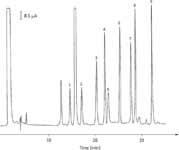
Figure 2: Representative chromatogram of the dabsyl derivates of amines from a standard mixture. For chromatographic conditions, see Experimental. Peaks: 1 = tryptamine, 2 = phenylethylamine, 3 = putrescine, 4 = cadaverine, 5 = histamine, 6 = internal standard (1,7-diaminoheptane), 7 = tyramine, 8 = spermidine, 9 = spermine.
In addition, analysts should evaluate the uncertainty associated with the chemical-calibration process. In this sense, two main sources of uncertainty can be distinguished: the calibration model used for the transformation of the analytical signal in concentration and the uncertainty associated with the normal distribution of the analytical signal.
These sources of uncertainty (intrinsic plus chemical calibration), which affect the analyte concentration in the sample, could be called inherent uncertainty. Furthermore, the definition of uncertainty (10) indicates that the presentation of results must be free from systematic effects. Thus, it is sometimes necessary to correct the results using recovery factors. When the recovery has been estimated, the bias must be checked to see whether it is statistically significant. This is done by determining whether this parameter is statistically different from 1, within the limit of its uncertainty, using a significance test of the form
where ta/2n is the two-sided t-tabulated value for the effective degrees of freedom associated withuR , which is the uncertainty of the estimated recovery R (11). uR was calculated as the standard deviation of recovery factors divided by the square root of the number of replicates.
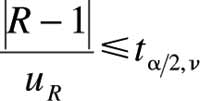
Finally, in our opinion, the main sources of uncertainty of an analytical method that must be identified and evaluated are working, inherent, and those resulting from the bias error associated with the method.
Quantify uncertainty components: The uncertainty associated with each potential source identified previously is estimated as standard deviation. It can be measured directly, calculated using previous experimental results, or obtained from theoretical studies.
Calculate overall uncertainty: The different contributions to the overall uncertainty must be combined according to the appropriate rules (2) to give an overall standard uncertainty. For a given equation involving only addition and/or subtraction, for example, y 5 r 1 s 2 t, the overall uncertainty is given by
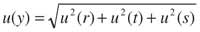
On the other hand, where multiplication or division are considered, for example, y 5 (rt )/ s, the overall uncertainty comes from the following equation:
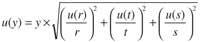
The statistical evaluation of the existence of bias by means of the t-test described previously permits the identification of two situations:
Bias not significant: No correction is applied, and the uncertainty associated with recovery should be included in the overall uncertainty for the final result using the equation
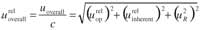
where c is the estimated result and is calculated by

Bias is significant: In this situation, depending upon the correction of the results, two approaches can be followed: When the results are corrected ccorr 5 c/R, the overall uncertainty calculated by equation 4 must be corrected and expressed as bearing in mind that this term is defined as
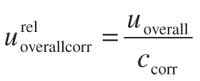
When the results are not corrected, uRdoes not represent the dispersion of the values properly, meaning the relative value of the bias must be considered as a new contribution to the overall uncertainty. Also, uR must be increased substantially in different ways, being denoted as uR 9 and estimated as
|1 2 R|/ t, where t is the critical value used in the significance test (12); according to Hã²³elbarth (13) by the following equation
121/R (4).
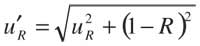
Expanded overall uncertainty: To provide a level of confidence for the final result, the expanded uncertainty is obtained by multiplying the overall standard uncertainty by a coverage factor k for which a value of 2 usually is chosen to obtain a confidence level of ~95%.
Results and Discussion
The different aspects considered previously for the estimation of uncertainty have been applied to the chromatographic determination of biogenic amines in liquid and solid samples.
Specifying the measurand: The analyte concentration is obtained from the equation
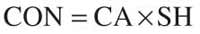
where CA is the analyte concentration obtained from the calibration curve in milligrams per liter, and SH includes the different treatments and dilutions of the sample, which can be expressed as
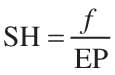
where f is the volumetric correcting factor for the dilution performed in the sample fitting-out to the measure step, and EP is the quotient between the sample mass ms and the volume from which ms has been extracted. For liquid samples where there is no extraction, EP is considered as 1, and its contribution to the overall uncertainty is zero.
Identifying uncertainty sources: The different contributions to the overall uncertainty associated with the analytical method applied are shown in an Ishikawa diagram (14) in Figure 3. It can be seen that for an unbiased determination, overall uncertainty arises mainly from working and inherent contributions.
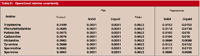
Table III: Operational relative uncertainty
Quantifying uncertainty components: In this step, each source must be converted to a standard uncertainty. For the basic equipment, the u values were calculated from the manufacturers' specifications, taking into account that for the microbalance, it was obtained directly and for volumetric material (pipettes and volumetric flasks), it was calculated by dividing the specified tolerance by 61/2 (2), because a triangular distribution was considered.
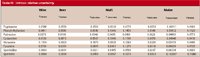
Table IV: Intrinsic relative uncertainty
Operational or working uncertainty: (uop)
The main contributions were evaluated from the following:
- preparation of stock solutions: (ustock-sol );
- preparation of the calibration solutions: (ucal-sol );
- sample handling: (uSH );
- preparation of dabsylation reaction: (udabs ).
Preparation of the stock solutions: ustock-sol.As can be seen in Figure 3, ustock-sol depends upon, among others factors, the purity of the standards. Since the manufacturers do not supply any uncertainty concerning this issue, the methodology based on van Look and Meyer (15) was applied here. For purities based on titration, they suggest using a rectangular distribution with a range from the minimum grade of purity indicated by the manufacturer (Pmin ) to 101%. The expected purity value is the mean, and the standard uncertainty of the purity u( P) is 29% of the range. Purities determined by chromatography can be described by a triangular distribution (ramp function). One leg of the triangle represents the range from Pminto 100%, and the right angle is located at 100%. The expected value is the median and the uncertainty u(P) is 24% of the range. Purities of the individual crystalline substances and their estimated uncertainties are shown in Table II.
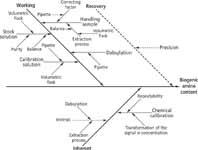
Figure 3: Cause and effect diagram (Ishikawa diagram) for the chromatographic determination of biogenic amines.
For the preparation of the stock standard solution, about 40 mg ( m) of crystalline substance (see Table II for accurate masses) were weighed into tin weighing boats. The weighed substances were put in a 50-mL (VF1) calibrated flask. The uncertainties of the microbalance and volumetric flask were 0.11 mg and 0.049 mL, respectively. Thus, the concentration of the stock solution is given by
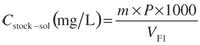
where P is the purity of the biogenic amine given as mass fraction, and 1000 is the conversion factor from milliliters to liters.
The relative standard uncertainty for the concentration of the stock solution is calculated by
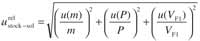
obtaining the values shown in Table II.
Preparation of the calibration solutions: ucal-sol. For this purpose, an intermediate stock solution was prepared by drawing an aliquot of 1.0 mL, VP1, (uVP1 5 0.004 mL) from the stock standard solution prepared previously into a 50-mL, VF2 , volumetric flask (uVF2 5 0.049 mL). The different calibration points were prepared by drawing different aliquots VP2 , (uVP2 5 0.004 mL) of this solution into five 25-mL, VF3 , (uVF3 5 0.012 mL) volumetric flasks. The relative uncertainty associated with this step can be obtained as
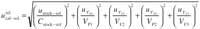
As a representative example of the calculations, Table III shows the uncertainty values obtained for the middle point of the calibration line.
Sample handling: uSH. As expressed in equation 9, there are two sources of uncertainty for the sample preparation. They are as follows:
Volumetric correcting factor:uf. Depending upon the type of sample analyzed (liquid or solid), the uncertainty associated with the volumetric correcting factor is different. An aliquot of 10 mL (u105 0.031 mL) is drawn to 50 mL (u50 5 0.020 mL) for liquid samples and to 25 mL (u255 0.012 mL) for solid samples. The values of uncertainty obtained are 0.0031 and 0.0031 for liquid and solid samples, respectively.
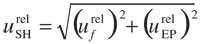
Extraction process: uEP. . The standard uncertainty is calculated, taking into account that 5 g of solid sample (ubalance 5 0.11 mg) is extracted with 50 mL of extractant (u50 5 0.020 mL), so the relative standard uncertainty is 0.000523. For liquid samples, this uncertainty is zero.
Therefore, the uncertainty due to the sample preparation is calculated from
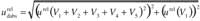
obtaining a value of 0.0031 for liquid samples and 0.0031 for solid samples.
Dabsylation reaction: udabs. As can be seen in Figure 1, the uncertainties for the added volumes can be calculated bearing in mind the standard uncertainty of each pipette is used. Following the appropriate rules, the uncertainty associated with this step is estimated by the equation

Table V: Relative chemical calibration uncertainty
obtaining a value of 0.0023.
The results obtained for the working uncertainty are shown in Table III.
Inherent uncertainty: uinherent. Intrinsic uncertainty: uintrinsic: This uncertainty primarily is affected by two factors not controlled by the chemist: dabsylation reaction and extraction. Their contributions will be different for solid and liquid samples. Thus, for liquid samples, uintrinsic is equivalent to udabs-chem, and for solid samples, it is calculated using the following equation:

Dabsylation reaction uncertainty: udabs-chem. For liquid samples, this uncertainty was estimated using replicate analysis of spiked samples (for the amines that were not detected) before the dabsylation reaction, and the uncertainty was expressed as standard relative deviation.
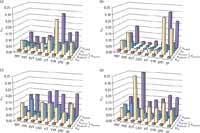
Figure 4: Contribution of the different sources to the relative uncertainty for the analytical determination of biogenic amines: (a) wines, (b) beers, (c) malt, and (d) maize.
For solid samples, uncertainty was estimated for the amines detected (using only one extract) and then the dabsylation procedure was performed on different aliquots of this. The value of udabs-chem is similar to those obtained for liquid samples, so it was not considered necessary to continue this study for the amines, which were not detected in solid samples. The results obtained under repeatability conditions are shown in Table IV, where to obtain relative uncertainties, the standard deviations were divided by their corresponding concentrations.
Extraction uncertainty: uextract-chem. The contribution of the extraction step to intrinsic uncertainty was estimated by analyzing spiked solid samples with detected and undetected analytes from different sample sizes under repeatability conditions. The uncertainty associated with these experiments included the contributions from the extraction process and the dabsylation reaction. For this reason, was estimated as the difference between the relative standard deviation of this process and the value established previously, according to the next equation

The results obtained are shown in Table IV.
Chemical calibration uncertainty: uchem-cal.Chromatographic determination uncertainty: uchrom-det:Since the concentration of biogenic amines is calculated using a calibration curve, the uncertainty depends on the transformation of chromatographic signals in concentrations. During the in-house validation process, and to obtain bias-free analytical results, we determined whether there was a matrix effect. Because the samples were not standard reference materials, this effect was studied on the basis of a statistical protocol (16,17) based on three calibration procedures: external calibration, internal calibration, and a calibration with different sample sizes (Youden calibration).
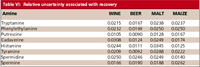
Table VI: Relative uncertainty associated with recovery
If the matrix effect is not detected, the analyte concentration could be obtained from the external calibration, correcting the constant bias for the analytes in which the Youden calibration detected its presence (16). In this case, five calibration levels were chosen for a linear response of the peak area versus the concentration of biogenic amine, with three replicates for each concentration. Therefore, the uncertainty due to the transformation of chromatographic signals in concentrations is estimated by applying the expression for the linear regression of least squares of residuals (18)
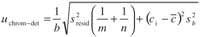
where b is the slope of the calibration curve, sbis its standard deviation, sresid is the standard deviation of residuals, ci corresponds to the mean concentration value of them sample replicates, and is the average concentration.
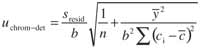
However , if a matrix effect is detected , the analyte content must be obtained from the standard addition methodology ( internal calibration ), and the uncertainty can be obtained using this expression ( 19 )where y¯ is the average response in the calibration and the other parameters have the same meaning as in equation 17.
Bearing in mind that these two uncertainties are given in absolute values, they must therefore be divided by the average concentrations to obtain the relative uncertainties, which are shown in Table V for the different types of samples.
Replicate measurements uncertainty: urep-cal. On the other hand, we consider that each replicate also can be transformed individually into a concentration (ci ) with an associated standard deviation (si ). Focusing on the si values obtained, they can be considered terms of a normal distribution of individual standard deviations. The width of the range depends upon two factors. The first is the variability of the standard deviations, and the second is the number of replicates. In our opinion, the mathematical relationship between the width of this normal s-distribution of the individual concentrations evaluated for a specific contribution of uncertainty of calibration model (m 5 1 in equation 17)
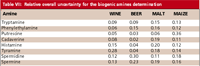
Table VII: Relative overall uncertainty for the biogenic amines determinationis the standard deviation of the s-mean (20). In this sense, the uncertainty urep-cal is evaluated using the following equation:
in which sdistribution is the width of the standard deviations distribution, and r is the number of replicates used. This type of uncertainty cannot be evaluated if there is a matrix effect, as shown in equation 18.
Finally, the chemical calibration uncertainty is evaluated according to equation 20:
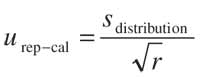
The results obtained are shown in Table V, where it can be seen that urep-cal is less important than uchrom-det .

Correction of the results: Contribution of bias to uncertainty: A recovery study was made to establish whether there was a systematic error associated with the analytical method. The samples (liquid and solid) were spiked with detected and undetected amines before sample treatment.
For the chromatographic determination of biogenic amines, the test explained previously (equation 1) was not significant in any case. Table VI shows the values of uR obtained.
Calculate overall uncertainty: The total uncertainty, termed relative-overall-standard uncertainty , is calculated according to equations 4 or 6 depending on the bias and the correction (or not) of the result. The results obtained for liquid and solid samples are shown in Table VII.
Expanded overall uncertainty: The contribution of the different sources to uncertainty is shown in Figure 4. As can be seen, the uncertainty for biogenic amines determination, expressed as standard deviation, ranged from 5% to 28% (wines), 3% to 30% (beers), 6% to 20% (malt samples), and 11% to 36% (maize samples).
The influence of working and recovery uncertainties is negligible, with those related to chemical aspects of the method uinherent being the dominant effects. Bearing in mind its two components (uintrinsic and uchem-cal), the influence of each depends upon the concentration of the amines in the sample. Thus, for low levels of the analytes in the sample analyzed, the contribution of calibration becomes the most important, whereas for high levels, the contribution of both terms is similar.
There are some differences between uintrinsic values for solid and liquid samples. This fact can be attributed to the double contribution arising from the dabsylation reaction and the extraction process.
Although the dabsylation reaction contribution is present in all the samples, the extraction process contributes significantly in solid samples. Thus, uintrinsic is higher in these.
Table VIII shows the results obtained for three samples (wine, beer, and malt), and the expanded overall uncertainty associated with each sample, indicating that putrescine and cadaverine are the amines with the lowest uncertainty associated with the results.
The methodology used to obtain the uncertainty makes it possible to identify the main uncertainty contributions, find their origin, and reduce them. In this chromatographic method, the main problems arise from two specific stages of the procedure: the sample handling and the calibration step, both of which are closely related to the chemical performance included as inherent uncertainty.
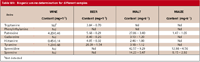
Table VIII: Biogenic amine determination for different samples
Conclusion
The estimation of the uncertainty of a chromatographic method has been discussed here using the data obtained from in-house validation and the bottom-up approach. Furthermore, the presence or absence of a matrix effect is considered, as is the presence of a bias in the analytical determination. A new source of uncertainty has been defined, termed inherent, and is associated with the chemicals aspects of the method. This allows us to decide whether it is necessary to redevelop or reoptimize the analytical method in order to reduce the uncertainty associated with the analytical results.
Acknowledgements
The authors would like to thank the ConsejerÃa de Educación y Ciencia de la Junta de AndalucÃa, Seville, Spain, for financial assistance (Research Groups FQM 0232 and RNM 0179, Research Project BTE2000-1493). They also would like to thank Robert Abrahams for editing the English version of the text.
References
(1)
Guide to the Expression of Uncertainty in Measurement
(International Organization for Standardization, Geneva, Switzerland, 1993).
(2)EURACHEM/CITAC Guide , S. L. R. Ellison, M. Rösslein, and A. Williams, Eds., (EURACHEM, Berlin, Germany, 2nd Ed., 2000), Available online:
(3) R.J.N. Bettencourt da Silva, M.J. Lino, J.R. Santos, and M.F.G.F.C. CamõesAnalyst 125, 1459-1464 (2000).
(4) L. Cuadros-RodrÃguez, M.E. Hernández-Torres, E. Almansa-López, F.J. Egea-Gonzà«¥z, F. J. Arrebola-Liébanas, and J.L. MartÃnez-Vidal, Anal. Chim. Acta 454, 297-314 (2002).
(5) V. J. Barwirck, J. Chromatogr. A 849, 13-33 (1999).
(6) D.B. Hibbert, J. Jiang, and M.I. Mulholland, Anal. Chim. Acta 443, 205-214 (2001).
(7) R. Romero, M.G. Bagur, M. Sánchez-Viñas and D. Gázquez, Chromatographia 51, 404-410 (2000).
(8) R. Romero, D. Gázquez, M.G. Bagur, and M. Sánchez-Viñas J. Chromatogr. A 871, 75-83 (2000).
(9) R Romero, M. Sánchez-Viñas and D. Gázquez, M. G. Bagur, and L. Cuadros RodrÃguez, Chromatographia 51, 481-484 (2001).
(10)International Vocabulary of Basic and General Standard Terms in Metrology , (International Organization for Standardization, Geneva, Switzerland, 1993).
(11) A. Maroto, R.Boqué Riu, and F.X. Rius, Anal. Chim. Acta 440, 171-184 (2001).
(12) M. Thompson, S.L.R. Ellison, A. Fajgelj, P. Willetts and R. Wood, Pure Appl. Chem. 71, 337-348 (1999).
(13) W. Hässelbarth, Fresenius' J. Anal. Chem. 365, 574-576 (1999).
(14) S.L.R. Ellison, and V.J. Barwick, Analyst 123, 1387-1392 (1998).
(15) G. van Look and V.R. Meyer, Analyst 127, 825-829 (2002).
(16)L. Cuadros-RodrÃguez, A.M. GarcÃa-Campaña, F. Alés-Barrero, C. Jiménez-Linares, and M. Román-Ceba, J. AOAC Int. 78, 471-476 (1995).
(17)A.M. GarcÃa-Campaña, L. Cuadros-RodrÃguez, J. Aybar-Muñoz, and F. Alés-Barrero, J. AOAC Int. 80, 657-664 (1997).
(18) L. Cuadros-RodrÃguez, A.M. GarcÃa-Campaña, and J.M. Bosque-Sendra, Anal. Lett. 29, 1231-1239 (1996).
(19) J.N. Miller and J.C. Miller, inEstadÃstica y QuimiometrÃa para QuÃmica AnalÃtica , I. Capella, Ed. (Pearson Education S. A., Madrid, Spain, 2002), pp. 129-130.
(20) J.N. Miller and J.C. Miller, inEstadÃstica y QuimiometrÃa para QuÃmica AnalÃtica , I. Capella, Ed. (Pearson Education S. A., Madrid, Spain, 2002), pp. 29-30.

Navigating The Path To Modern Slalom Chromatography: An Interview with Fabrice Gritti
May 1st 2025Fabrice Gritti, consultant scientist at Waters Corporation, spoke to LCGC International about the history of slalom chromatography (SC) and why he decided that the technique was worth re-investigating. The potential benefits of SC, according to Gritti, include identifying RNA impurities when manufacturing mRNA therapeutics, and assisting in the development of new gene and cell therapies, and other biopharmaceutical applications.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)




