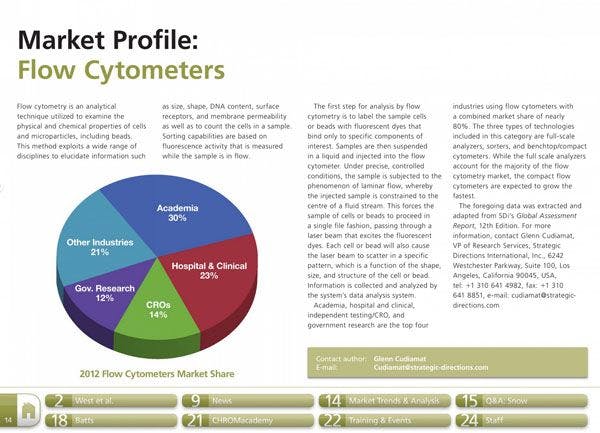
Speeding up Biopharmaceutical Separations using Ion-Exchange Chromatography
The efficiency of protein separation by ion-exchange chromatography could be increased by up to a factor of 5 according to a new study, potentially speeding up biopharmaceutical production.1 Christy Landes of Rice University in Texas (USA) and her team performed the first molecular-scale investigation into protein ion-exchange chromatography by using direct super-resolution techniques and the stochastic theory.
The efficiency of protein separation by ion-exchange chromatography could be increased by up to a factor of 5 according to a new study, potentially speeding up biopharmaceutical production.1 Christy Landes of Rice University in Texas (USA) and her team performed the first molecular-scale investigation into protein ion-exchange chromatography by using direct super-resolution techniques and the stochastic theory.
Ion-exchange chromatography is the primary technique used to separate proteins of interest from extracts during the manufacture of biopharmaceuticals, but it represents a “bottleneck” within the production process. Understanding the interactions between the mobile and stationary phases and using the stochastic theory of chromatography could enable tailoring of the stationary phase and therefore improve efficiency.
The project began with a hypothesis generated with collaborators in the Wilson group at the University of Houston. Landes told LCGC that the team believed that there was more to ion-exchange chromatographic separations than just electrostatic interactions; rather, that it depended on the specific clustering of adsorbents in a column. Landes said: “As usually happens with a great project, our need drove us to innovate. The goal to understand single protein adsorption at a realistic separations support caused us to look to the recent field of super-resolution imaging, but to expand that to measure the dynamics of proteins interacting with ligands.”
The team had already developed a super-resolution technique called mbPAINT (“motion blur” point accumulation for imaging in nanoscale topography) for mapping the capture and release of single DNA strands.3 DNA probes labeled with fluorescent tags were passed over immobilized single stranded DNA. On binding, even if just for a short time, a bright blur would be captured. Careful analysis of the images allowed the visualization of molecules smaller than the natural diffraction limits of light-based imaging.
Instead of using single stranded DNA, ligands were constructed on an agarose support matrix film. Peptides labeled with a fluorescent probe were passed over the ligands, and, on the formation of an interaction, released a signal that was captured by a camera. No more than a blur, multiple images were processed against the background distortion allowing the pinpoint of the location of the interaction. The interactions between single proteins and individual charged ligands were then quantified.
The paper demonstrated that clusters of charges are required to create adsorption sites and that even homogenously charged ligands can create alternate kinetics if the availability of the ligand is altered. Furthermore, the data was related to the stochastic model to suggest that clustering could be optimized by purposefully engineering ligand positions that currently exist accidentally.
When asked about the long-term importance of the results, Landes told LCGC: “Given the highly empirical methods currently used in the separations industry, and given the incredibly high and always rising cost of separations, we believe that molecular-scale insight such as provided in our paper could have a very high impact.” She added: “Any time a process occurs on a hundred billion dollar scale, as does the global pharmaceutical market, even small improvements can lead to serious savings. And that was one reason we included simulated separation curves in our work: They suggest that a 500% improvement could be made just by optimizing the clustering that currently occurs only by accident.”
References
1. L. Kisley, J. Chen, A.P. Mansur, B. Shuang, K. Kourentzi, M-V Poongavanam, W.H. Chen, S. Dhamane, R.C. Wilson, and C.F. Landes, PNAS 2014; published ahead of print January 23, 2014, DOI: 10.1073/pnas.1318405111
2. J. Chen, A. Bremauntz, L. Kisley, B. Shuang, and C.F. Landes, ACS Appl. Mater. Interfaces5(19), pp 9338–9343 (2013).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)