SEC Method for Cellulose Acetates of Wide DS-Range
Tosoh Bioscience Application Note
Cellulose acetate (CA) is one of the most important cellulose derivatives. Its main applications are their use in membranes, films, fibres, plastics and filters. The chemical and physical properties of these derivatives are strongly influenced by the degree of substitution (DS) and the molar mass and molar mass distribution. Therefore, there is a strong need to characterize these properties.
SEC provides a convenient way for the characterization of molar masses and molar mass distribution of synthetic and natural polymers. However, a crucial balance of the polarities of the analyte, the solvent and the stationary phase is required for a reliable SEC method. Since DS of cellulose derivatives alters the polarity, solubility and aggregation behaviour, the SEC methods found in literature are usually valid only within a narrow DS-range. Published SEC methods usually apply THF or DMF modified by the addition of LiBr as eluents on styrene-divinylbenzene (SDV) columns. However, often neither the degree of substitution nor the DS-range applicable for the given chromatographic conditions are specified. The only SEC system suitable for cellulose acetate over a wide DS-range (DS range 0.4-1.2) uses pyridine/water on a GRAMS column. This application note reports on SEC-separations of high molar mass CAs for CAs of higher DS in the range DS = 1.5-3.
Most samples were prepared by partial saponification of a commercial CA sample with a DS of 2.6. Other samples were of industrial origin. A Tosoh Biosystems EcoSECSystem (Tosoh, Stuttgart, Germany) equipped with a MALLS detector (DAWN DSP, Wyatt Technology, Santa Barbara, USA) was used. The stationary phase was a GRAM linear XL column (10 µm particle size, 30 × 0.8 cm, PSS Polymer Standards Service, Mainz, Germany). DMSO was used as the eluent. The low refractive index increment of cellulose acetates in DMSO (0.024-0.044 mL/g, depending on DS) does not allow for reliable molar mass determination by SEC-MALLS. The very sensitive EcoSEC RI, however, could easily detect those samples reliably. However, the light scattering instrument revealed aggregation if no or only low LiCl concentrations were used. Aggregation was not observed at LiCl concentrations above 50 mmol/L. Some of the samples required higher salt concentrations for dissolution.
Figure 1 shows a comparison of the chromatograms of three CAs of different DS, which were prepared from the same base material. The elution volumes and the peak shapes of the samples are nearly identical, indicating that no degradation occurred during saponification. This has also been confirmed by molar mass determination using light scattering in a different solvent with higher sample contrast (dn/dc). The close agreement in the chromatograms of the samples can therefore be regarded as an indication of nearly identical calibration curves of the cellulose acetates despite differences in DS. The fourth sample of DS = 1.72 is a commercial sample. It is presented in order to show that the close agreement of the chromatograms is not due to an insufficient chromatographic resolution. The independence of the calibration curves on DS is advantageous. It will allow reliable molar mass determination of cellulose acetates without worrying about variations of SEC elution volume due to differences in DS.
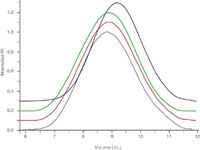
Figure 1: Stacked overlay of chromatograms of cellulose acetates of different DS: DS (grey: DS = 2.45, red: DS = 2.09, green: DS = 1.59) derived from the same base material by partial saponification and a commercial sample (blue: DS = 1.72).
Tosoh Bioscience
Zettachrgin 6, 70567 Stuttgart, Germany
tel:+49 (0)711 13257-0
fax:+49 (0)711 13257-89
E-mail: info.tbg@tosoh.com
Website: www.tosohbioscience.com
Reference
1. Soo-Jung Lee et al., Carbohydrate Polymers, 54, 353-362. (2003)

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.













