Retention-Time Variations Minimized Using UPLC-MS
Scientists from Fudan University in Shanghai, China created a system for reducing retention-time variations in metabolomic analysis using ultrahigh-performance liquid-chromatography coupled with mass spectrometry (UPLC-MS).
Scientists from Fudan University in Shanghai, China developed a method for reducing retention-time variations in metabolomic analysis using ultrahigh-performance liquid-chromatography coupled with mass spectrometry (UPLC-MS). The team’s research was published in Talanta (1).
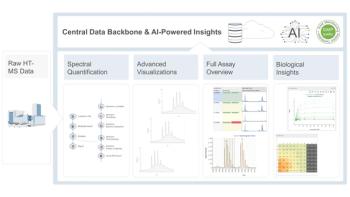
Reliable metabolite identification must involve reproducible data for MS and retention-time (tR). However, inter-experiment tR variations are usually found due to column deterioration. To compare analytes’ tR from different experiments, retention index (RI) is often used. In this study, the scientists created an endogeneous retention-index (endoRI) from straight-chain acylcarnitines in biological samples. This allowed for a quantitative relationship between acylcarnitines’ tR and chain-length (LC) for tR prediction. Additionally, the effectiveness of endoRI-corrections of tR variations was evaluated alongside multiple factors, including changes in mobile phase, elution time, flow-rates, and column temperatures were tested along multiple biological samples, including human plasma, serum, urine, and animal liver samples.
The endoRI-corrections were found to reduce tR variations caused by the changes by up to 5.1min–0.2min for most metabolites. In the above biological samples, inter-batch and inter-platform variations were reduced from 1.5 min to 0.15 min for 95% of detected features. The results of this study enabled the establishment of a quantitative model between tR and LC for predicting tR values of acylcarnitines outside of samples. This allows data from different databases and batches to be compared to each other.
Reference
(1) Chen, Q.; Lu, Q.; Zhang, L.; Zhang, C.; Zhang, J.; Gu, Y.; Huang, Q.; Tang, H. A Novel Endogenous Retention-Index for Minimizing Retention-Time Variations in Metabolomic Analysis with Reversed-Phase Ultrahigh-Performance Liquid-Chromatography and Mass Spectrometry. Talanta 2024, 268 (1), 125318. DOI:
Newsletter
Join the global community of analytical scientists who trust LCGC for insights on the latest techniques, trends, and expert solutions in chromatography.