Quantitative Comparison of Hormones in Drinking Water Between Low and High Resolution Mass Spectrometry
The Column
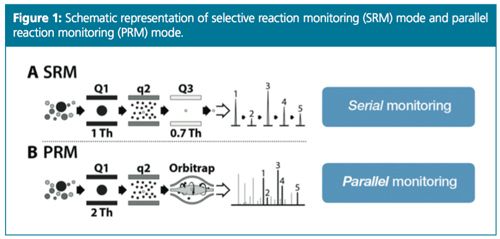
The quantitative performance of the latest generation of high-resolution instruments is comparable to that of a triple quadrupole MS, even though different scanning modes are used. Higher-resolution instrumentation also allows flexibility concerning compound identification because the experiment can be set up for targeted quantitation, screening, or both. In an Orbitrap-based instrument, the parallel reaction monitoring (PRM) mode performs most closely to a triple quadrupole mass analyzer using selected reaction monitoring (SRM) mode. This study looks at the performance of an Orbitrap-based LC–MS method for EPA Method 539.
Photo Credit: Atomic Imagery/Getty Images

Ali Haghani,1 Andy Eaton,1 Richard F. Jack,2 Claudia P.B. Martins,2 and Dipankar Ghosh,2
1Eurofins Eaton Analytical, Inc., Monrovia, California, USA, 2Thermo Fisher Scientific, San Jose, California, USA.
The quantitative performance of the latest generation of high-resolution instruments is comparable to that of a triple quadrupole MS, even though different scanning modes are used. Higher-resolution instrumentation also allows flexibility concerning compound identification because the experiment can be set up for targeted quantitation, screening, or both. In an Orbitrap-based instrument, the parallel reaction monitoring (PRM) mode performs most closely to a triple quadrupole mass analyzer using selected reaction monitoring (SRM) mode. This study looks at the performance of an Orbitrap-based LC–MS method for EPA Method 539.
Increasingly, contaminants of emerging concerns (CEC), which includes pharmaceuticals and personal care products such as the contraceptive pill and antibiotics, are being detected at low levels in surface water. Many of these CECs are compounds that can alter the normal functions of hormones and are known as endocrine disruptor compounds (EDCs); they can cause a variety of adverse health effects.1 For example, release of low concentrations of hormones has resulted in hermaphroditic fish that are unable to breed, resulting in a crash of fish populations in several lakes. As a result the EPA has developed Method 539 for the Unregulated Contaminant Monitoring Rule 3 (UCMR 3) programme, which collects data for contaminants suspected to be present in drinking water but that do not have health-based standards set under the Safe Drinking Water Act (SDWA).2
The identification and quantification of micropollutants at low concentrations requires both sensitivity and selectivity against complex matrices. Selected reaction monitoring (SRM) of precursorâproduct ion transitions that makes use of a triple quadrupole mass analyzer has been the method of choice in recent years because it’s the primary way to achieve accurate quantification. However, other screening strategies taking into account full scan mode and other advanced tandem mass spectrometry (MS–MS) scan modes can potentially offer a valuable alternative to SRM-based methods as a result of the development of a more rugged, sensitive, and selective instrumentation.
The latest generation of high resolution instruments can be compared in terms of quantitative performance to a triple quadrupole, even though different MS scanning modes can be used, depending on whether the experimental objectives are quantitation, screening, or both. Higher resolution instrumentation also allows for more flexibility in compound identification. Parallel reaction monitoring (PRM) mode is the closest scan mode to working with a triple quadrupole mass analyzer using SRM mode. This study compares different quantitative workflows applied to the standardized EPA method 539 between traditional MS–MS and an orbitrap highâresolution accurate mass spectrometry (HRAMS) instrument.
Experimental
Sample Preparation: 500 mL (instead of 1L) of a dechlorinated sample with omadine biocide was extracted through an SPE octadecyl (C-18) column (Thermo Fisher Scientific) after adding surrogates. The eluent from SPE was concentrated to dryness, and then diluted to 1 mL with 50/50% methanol/water. An aliquot was injected into the liquid chromatography tandem MS (LC–MS–MS) system (Thermo Fisher Scientific) after adding internal standards and quantified against the internal standard.
LC–MS Conditions: The EPA allows method flexibility for columns, eluents, and MS conditions in general. The following conditions were used: mass analyzer: Q-Exactive (orbitrap mass spectrometer) (Thermo Fisher Scientific); mass resolving power: 70000 FWHM; scan mode: PRM; AGC: 2e5; IT (ms): 200; isolation window (m/z): 1.0; HPLC: Thermo Scientific RS Ultimate UHPLC 3000, binary pump, autosampler, and column heater with 100 μL sample loop (Thermo Scientific); Column: Acclaim Polar Advantage II (2.1 × 150 mm, 3-μm 120Å, Thermo Fisher Scientific); eluents: A) 1 mM ammonium fluoride in water, B) 50:50 (v/v) acetonitrile/methanol, gradient flow at 0.3 mL/min with a 21.4 min run; injection volume: 50 μL.
The EPA Method 539 uses a triple quadrupole method using an SRM scan mode (also known as MRM). In this study a similar set of conditions was used.4
In parallel reaction monitoring (PRM) mode a list of targeted precursor ions, retention times, and collision energies can be included in the method. When detecting a targeted ion, the system isolates that precursor ion in the quadrupole and triggers MS–MS experiments, generating MS–MS spectra that can be used for both quantitation and identification. Both the quantitation and identification are performed taking into account product ions generated after the isolation of a specific precursor ion. This operating mode is similar to an SRM (or MRM) experiment using a triple quadrupole instrument. In PRM mode, the third quadrupole is substituted with a HRAMS analyzer, enabling the parallel detection of all target product ions (Figure 1).
The number of scans across the chromatographic peak is dependent on the cycle time of the instrument and therefore on the set of conditions used (for example, resolving power). These conditions can and should be optimized depending on the objectives of the experiment. In this case, accurate quantitation as well as unambiguous identification has been targeted.
Requirements: The EPA has strict requirements that should be met before the analysis of any sample. These are known as initial demonstration of capability (IDC). IDC includes the demonstration of low background noise, precision by analyzing four to seven extracted laboratory fortified reagent water blanks (LFB) at mid-level, the demonstration of accuracy, and, finally, the demonstration of capability necessary to meet the minimum reporting limit (MRL).
Results
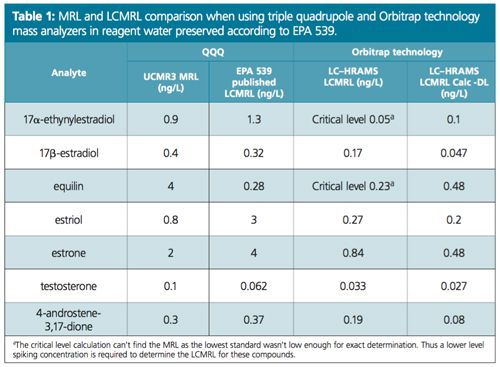
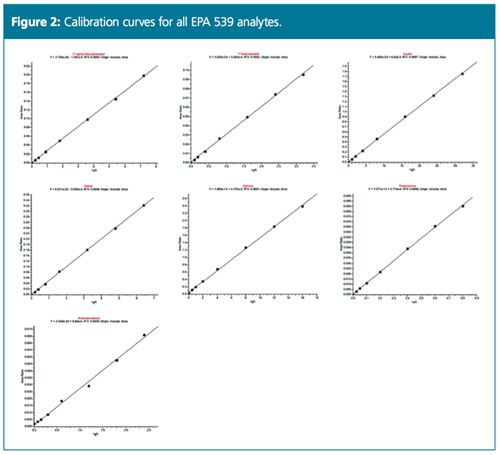
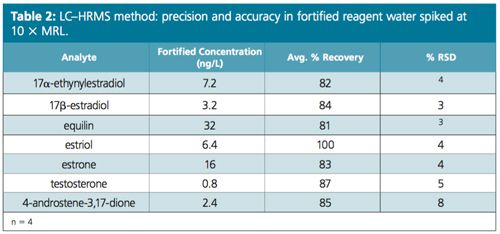
Excellent linearity was demonstrated from a range starting at ¼ of MRL (Figure 2). Table 1 compares the MRL and the lowest concentration minimum reporting level (LCMRL) obtained when using both SRM and PRM modes. Tables 2, 3, and 4 summarize the precision and accuracy of the method after the LC–HRAMS analysis of different types of samples–reagent water spiked at different levels and unregulated contaminant monitoring rule 3 (UCMR3) water samples.
As shown in Table 1 the LCMRL and DL were much lower when using LC–HRAMS than the detection limits reported in the EPA 539 method. In order to demonstrate method robustness, the EPA requires the demonstration of performance using a fortified matrix in blanks, reagent water, and real samples. Results are summarized in Tables 2, 3, and 4.
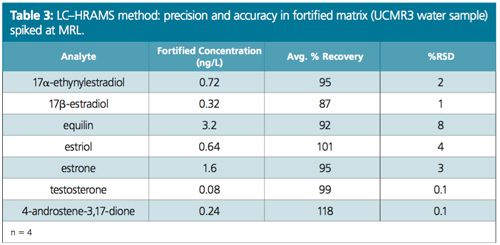
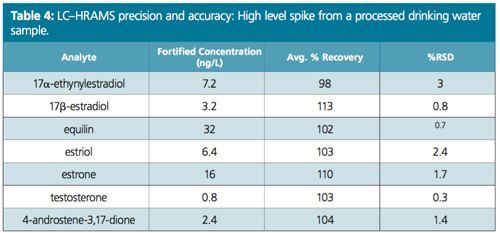
Conclusion
The LC–HRAMS method proved to be sensitive, accurate, and reproducible, a reliable alternative to the use of triple quadrupoles in the quantification of hormones in drinking water according to the EPA guidelines. By using different scanning modes within the orbitrap MS–MS, quantitation on precursor ions and identification of fragments ions are possible. These scanning modes do not infringe on the requirements in many regulated methods and can potentially be used for compliance monitoring. LC–HRAMS can offer sensitivity and selectivity in the quantitation of known contaminants in drinking water, while potentially enabling the combination of targeted and non-targeted analysis of unknown contaminants in the same run.
References
- https://www.epa.gov/wqc/contaminants-emerging-concern-including-pharmaceuticals-and-personal-care-products (last accessed 15 March 2016)
- https://www.epa.gov/sdwa
- S.D. Winslow, B.V. Pepich, J.J. Martin, G.R. Hallberg, D.J. Munch, C.P. Frebis, E.J. Hedrick, and R.A. Krop, Environ. Sci. Technol. 40, 281–288 (2006).
- EPA Method 539 “Determination of Hormones in Drinking Water by Solid Phase Extraction (SPE) and Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry LC-ESI-MS/MS)” version 1.0 (2010)
- Unregulated Contaminant Monitoring Rule 3 (UCMR3) http://water.epa.gov/lawsregs/rulesregs/sdwa/ucmr/ucmr3/
- S.D. Richardson and Th. A. Ternes, Anal. Chem.86, 2813–2848 (2014).
Andy Eaton is currently the Technical Director and Vice President of Eurofins Eaton Analytical (EEA), the largest potable water testing lab in the USA. He has been at EEA for nearly 35 years. His lab has performed UCMR3 testing for more than 500 PWS across the US, and serves as a contract lab for EPA for small system UCMR3 analytical testing. EEA perform method validation and LCMRL determinations for EPA for many of the UCMR3 methods. He serves on several American Water Works Association (AWWA) Technical Advisory Workgroups and co-authored AWWAs comments on each of the UCMRs. He is a member of the Joint Editorial Board of Standard Methods for the Examination of Water and Wastewater. He earned a PhD from Harvard in geochemistry and performed postdoctoral work at Caltech in environmental geochemistry.
Ali Haghani is the Research and Development Manager of Eurofins Eaton Analytical Inc. He has over 15 years of experience in metals, GC, GC–MS, wet chemistry, and ion chromatography. He has assisted the EPA with method validations on numerous UCMR3 methods and co-authored EPA method 314 for perchlorate. Mr. Haghani is responsible for methods development, training,
and instrument troubleshooting throughout the laboratory. He has developed state of the art methods for EDCs/PPCPs and PFCs.
Richard F. Jack is the Senior Director, Vertical Marketing - Environmental and Industrial.
Claudia P.B. Martins works in the Chromatography and Mass Spectrometry Division.
Dipankar Ghosh is the Director - Environmental Food Safety and Industrial Markets, LSMS.
E-mail: andyeaton@eurofinsus.com
Website: www.eurofinsus.com / www.thermofisher.com

Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
Studying Cyclodextrins with UHPLC-MS/MS
May 5th 2025Saba Aslani from the University of Texas at Arlington spoke to LCGC International about a collaborative project with Northwestern University, the University of Hong Kong, and BioTools, Inc., investigating mirror-image cyclodextrins using ultra-high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS) and vibrational circular dichroism (VCD).

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








