Quantitation and Characterization of Copper Plating Bath Additives by Liquid Chromatography with Charged Aerosol Detection and Electrochemical Detection
The Column
A novel approach is described for the quantitation of three additives typically used in copper plating using a dual LC system with an ECD and a CAD.
Copper plating using an acid bath is a process widely used in the manufacture of a variety of products. Different additives can be used to control different aspects of the copper plating process, and must be accurately quantified. In this article, an approach using a dual liquid chromatography (LC) system with an electrochemical detector (ECD) and a charged aerosol detector (CAD) is described for the quantitation of three additives typically used. The methods are precise and sensitive for the determination of all additives, giving a quantitative measure of suppressor and suppressor degradation. Calibration curves and sample analysis results are reported for all additives. Both analyses can be run using the same sample preparation and on the same system.
Copper plating describes the process of depositing a layer of copper onto the item to be plated using an electric current. It is used in the manufacture of a variety of products ranging from cooking pots to integrated circuits and satellite components. To maintain quality and consistency of plating, the process must be tightly controlled.

Photo Credit: YinYang/Getty Images
The acid copper plating bath method is one of the most common approaches used in copper plating. The main components include copper sulphate and sulphuric acid, in addition to a number of additives. The organic additives influence the quality of the copper deposition and typically include an accelerator (such as bis-(3-sodiumsulphopropyl) disulphide), a suppressor (a polyalkylglycol), and a leveller (either a large-molecular-weight polymer or small molecule containing nitrogen or sulphur). Each modifier has a particular function, determining speed of plating, surface wetting, and surface smoothing. To ensure quality control, the levels of each of these modifiers has to be tracked and controlled.
The most commonly used analytical method for measuring these additives is cyclic voltammetric stripping (CVS), which measures the combined effects of the additives in an iterative process using additive standard additions, which is reported to be very slow (analysis in hours) and not very accurate.1 We have developed a high performance liquid chromatography (HPLC) methodology that has been shown to quantify the majority of additives in copper plating bath samples.
Most modifiers do not possess a chromophore, and within the bath, the levels of accelerator and leveller are present at minute concentrations, therefore limiting the detectors available for quantitation. These HPLC methods can provide selective quantitation of these additives, without the use of sulphuric acid mobile phases,1,2 which cause rapid column deterioration.
A faster method to quantify additives is to perform LC using a dual-gradient HPLC system with two detectors, such as a charged aerosol detector (CAD) and an electrochemical detector (ECD). The ECD uses applied potentials to oxidize analytes, which provides the signal. Any analyte with an oxidizable group can be measured at very low amounts (typically picograms on column). It is effective for measuring low levels of electrochemically active analytes in complex matrices, such as the accelerator and leveller in an acid bath.
The CAD is a non-selective, mass-based detector that can measure nonvolatile analytes, with or without chromophores. The analyte is first converted into aerosol droplets using nebulization. The mobile phase is evaporated, leaving analyte particles that become charged in the mixing chamber. The charge is then measured by a highly sensitive electrometer. Charged aerosol detection is reported to have greater sensitivity and precision than evaporative light scattering (ELS) and refractive index (RI), and it is simpler and less expensive to operate than a mass spectrometer (MS).3 Analyte response is also largely independent of chemical structure, providing clear relationships among different analytes in a sample.
ECD uses coulometric working electrodes that are more sensitive and selective than traditional amperometric electrodes.4 The selectivity of serially placed coulometric electrodes is presented in Figure 1. The first electrode is typically held at a low potential, the second at a higher potential. As the analytes pass through from one electrode to the other, labile compounds will oxidize at the first electrode, leaving the second (downstream) electrode free to measure more stable compounds without interference. In the example shown in Figure 1, analyte A oxidizes to P on electrode 1 (E1) held at 100 mV applied potential, effectively removing it from further reaction. Analyte B remains unchanged until it encounters electrode two (E2) at 500 mV applied potential. At E2, analyte B oxidizes to Q. This provides a selective means of quantifying different analytes.
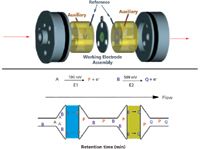
Figure 1: Functioning of electrochemical detection with coulometric cells, (top) a single cell and (bottom) in series.
Both detectors can be run simultaneously, and with this configuration the autosampler can be exchanged between the two systems without interrupting flow to either system.
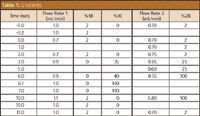
Table 1: Gradients.
Method
Liquid Chromatograph Set Up: HPLC system: UltiMate 3000 ×2 Dual-gradient UHPLC, equipped with dual-gradient DGP3600RS pump, TCC-3000RS with switching valve, WPS3000RS autosampler, and solvent degasser. Mobile phase 1A: 18.2 MΩ·cm water containing 2.5 g/L; sodium perchlorate and 2.5 mL/L (10% perchloric acid). Mobile phase 1B/2B: Methanol. Mobile phase 1C: Acetonitrile/methanol (900:100) with perchlorates as above. Mobile phase 2A: (70% Ethyl amine)/acetic acid in water (6 mL/L:4 mL/L), pH 5–6. Injection volumes: 100 μL. HPLC column 1: 4.6 × 150 mm, 2.6-μm, Thermo Scientific Accucore C18. HPLC column 2: 4.6 × 150 mm, 5-μm, Thermo Scientific Acclaim 120 C18. Column temperature: 40 °C. Detector 1: ECD3000RS with 6011RS Electrochemical cell; Electrode 1: +650 mV; Electrode 2: +900 mV, relative to Pd. Detector 2: Corona ultra RS Data rate: 10 Hz. Filter: 4. Power function: 2.00 (5.8–8.5 min). Sample temperature: 20 °C.
The HPLC system, data collection, and processing were all operated by and performed on the Thermo Scientific Dionex Chromeleon 7.2 software.
Sample and Standard Preparation: All copper plating bath standard solutions and samples must be properly neutralized prior to injection onto the HPLC system. Generally, samples are diluted with N,N-dimethylethanolamine to attain a pH value between 2 and 4: An aliquot of sample (750–950 μL) is placed into a glass HPLC vial, the amine is added, and the vial is capped and shaken vigorously. If the sample is not clear, the pH is likely too high, and the results may be inaccurate. Once the proper ratio of amine to sample is determined, standards are prepared in the bath matrix (no additives), dilutions are made, and the standard solutions are neutralized in the same manner.
Results
Analysis of Accelerator and Leveller by ECD: Standards of stock additive solutions were prepared in concentrations of 300% nominal concentration (NC), and serially diluted to low concentrations. Standards were analyzed in triplicate to determine calibration curves and instrument precision. The linear correlation coefficients were acceptable with R2 = 0.9987 and 0.9945 for accelerator and leveller, respectively. Peak area percent relative standard deviation (RSD) values ranged from 0.6 to 2.3 for accelerator and 4.9 to 18.6 for leveller, with the higher values at the low concentrations.
A copper plating bath sample containing accelerator, leveller, and suppressor was neutralized and analyzed by HPLC–ECD, with the two chromatograms shown in Figure 2. For spike recovery evaluation, the sample was diluted to 50% of initial concentration with either the matrix blank or the 100% NC standard solution to provide a 50%-NC spiked sample. The recovery values were found to be 99% for the accelerator, and 70% for the leveller. This leveller recovery value was confirmed using a second bath sample, and by the method of standard addition, indicating that the recovery value is stable. The signal-to-noise (S/N) value of the leveller (blue) in this spiked sample was 66, indicating that sufficient sensitivity was available for these determinations. Signal-to-noise values of 10 and 3.3 were used to calculate the limits of quantitation (LOQ) and detection (LOD), respectively. The LOQ and LOD values were 1% and 0.3% NC for the accelerator and 20% and 7% NC for the leveller, respectively.

Figure 2: HPLCâECD chromatograms, accelerator at +900 mV (black) and leveller (blue) at +650 mV, relative to palladium.
Accelerator and Suppressor Analysis by CAD: Standards of stock additive solutions were prepared in concentrations of 300% NC, and diluted to low concentrations. Injections were made in triplicate to determine calibration curves and instrument precision, respectively. At 100% NC, five samples were individually prepared and analyzed. The linear regression correlation coefficients were high with R2 = 0.9987 and 0.9957 for accelerator and suppressor, respectively. Precision RSD values, based on peak areas varied between 1.0% and 5.3% for the accelerator, and were less than 1% for the suppressor. The RSD precision values of the five individually prepared samples at 100% NC showed similar values using the CAD, indicating that sample preparation is reproducible.
In addition to method accuracy and precision data, the HPLC–CAD method was evaluated for spike recovery in a similar manner as presented for the ECD evaluation. Recovery values for the accelerator were 103%, and for the suppressor, 95–100%. Analyte resolution was also evaluated. Four peaks were resolved for this accelerator (not shown).
The sensitivity for the accelerator was found to be 3% NC for LOQ, based on a S/N ratio of 10. In the sample chromatograms shown in Figure 3, two plating bath samples are overlaid and consist of a new and a used plating bath. The suppressor is seen as the largest peak in the chromatograms, along with many smaller peaks with earlier retention times. These smaller peaks represent lower molecular weight fractions of the suppressor. Compared to the new bath, the suppressor concentrations differed by nearly sevenfold in the used bath, along with a fourfold increase in the relative amounts of the smaller molecular weight fractions. These changes are related to suppressor degradation as the bath is operated.5 By integrating the peak area of the smaller molecular weight suppressor and comparing this value to either the total suppressor peak area or the peak are of the original suppressor, a quantitative measure of the suppressor quality can be achieved.
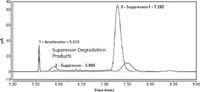
Figure 3: Overlaid HPLCâCAD chromatograms of new (black) and used copper plating bath samples.
As the plating bath is used, additives are consumed by the process and eventually replenished, as indicated by analytical determinations. For example, in some analyses (not shown), the amount of suppressor rapidly lost 73% of its original concentration, accelerator lost 50%, and the leveller lost 40% over 25 Ah/L of operation without replenishment.
In other experiments, it was found that the HPLC results obtained from these two methods correlated linearly with CVS data, as shown in Figure 4, though with differing y-intercept values. This difference in y-intercept has been attributed to the different properties of measurement, where HPLC measures the actual amounts and components of additives, and CVS measures the activities of the additives.6 This would drastically reduce the analysis time for a plating bath sample by several hours.
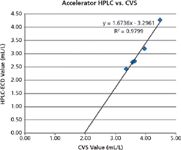
Figure 4: Linear correlation between orthogonal determinations of accelerator by HPLCâECD and by CVS.
Since the additive chemicals are typically of unknown concentration and structure, and plating baths can use a variety of chemicals to control their plating process, the analytical method will likely require modifications, and most typically for the leveller additive. The HPLC method can reliably measure accelerator and suppressor amounts within 30 min. Levellers can be measured by ECD (or CAD), but can be more difficult. In the cases where leveller cannot be detected, the quantities of accelerator and suppressor can be used in combination with CVS to determined leveller amounts in less time.
Conclusions
Two sensitive and fast methods using HPLC with the universal detection of CAD and the specific detection of ECD were used to determine the quantities of the three additives, accelerator, suppressor, and leveller. Both methods have sufficient sensitivity to accurately determine concentrations of all three analytes with a single sample preparation and in less than 30 min.
Another benefit of chromatography over CVS is that the quality of the bath can also be determined, mainly from the breakdown of the suppressor: As the bath is operated, a certain threshold of suppressor deterioration can be determined, after which the bath can be carbon-processed.
These methods, once adjusted to operate for a particular plating bath compostion, can be used for fast monitoring, and increased control of additive concentrations without high demand for personnel time. This will potentially reduce the likelihood of operator errors and production shutdowns, which can be very costly.
References
1. K.J. Hong, Korean Phy. Soc. 43(2), 286–289 (2003).
2. Application Note 139: Dionex, Part of Thermo Fisher Scientific. http://www.dionex.com/en-us/webdocs/4755-AN139_V16.pdf(Accessed 24 Jan 2011).
3. J.L. Wolfender, Planta Med. 75(7), 719–34 (2009).
4. I.N. Acworth et al., Eds., Coulometric Electrode Array Detectors for HPLC, (VSP, Utrecht, The Netherlands, 1997) pp. 382.
5. M. Pavlov, E. Shalyt, and P.A. Bratin, New Generation of CVS Monitors Cu Damascene Plating Baths. Electro IQ.[Online] http://www.electroiq.com/articles/sst/print/volume-46/issue-3/features/chemical-handling/a-new-generation-of-cvs-monitors-cu-damascene-plating-baths.html (accessed 24 Jan 2012).
6. R. Gluzman, P. Bratin, L. Fass, J. Rickly, and J. Rie, Plating Bath Analysis and Control by CVS (1992) http://infohouse.p2ric.org/ref/29/28351.pdf (last accessed 14 Mar 2014).
Marc Plante is an applications scientist at Thermo Fisher Scientific, focused on developing HPLC methods that use charged aerosol detectors. He earned his doctoral degree from Northeastern University, Boston, Massachusetts, USA, following his undergraduate studies in chemistry and engineering at Rensselaer Polytechnic Institute, Troy, New York, USA.
Bruce Bailey is a product applications manager at Thermo Fisher Scientific. He studied biology and chemistry and earned his doctoral degree at the University of Waterloo in Ontario, Canada. He has helped develop many commercial applications using electrochemical and charged aerosol detectors.
Ian Acworth is director and customer and applications support at Thermo Fisher Scientific. He studied biochemistry at Magdalen College, Oxford, UK, where he earned his BA, MA, and D. Phil. Degrees. He was a post-doctoral fellow at the Department of Brain and Cognitive Services, Massachusetts Institute of Technology, specializing in neuropharmacology.
E-mail: marc.plante@thermofisher.com
Website: www.thermoscientific.com/en/products.rapid-separation-rs-systems.html
This article is from The Column. The full issue can be found here:http://images2.advanstar.com/PixelMags/lctc/digitaledition/April28-2014-uk.html

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









