A Novel Sample Preperation Approach to Increase the Throughput of Pesticide Analysis by LC–MS–MS
The high-throughput analysis of pesticides in raw agricultural commodities has been hindered by the slow and laborious sample preparation stages. Using traditional clean-up procedures, as many as 20 steps are required to get the sample in a convenient form for analysis, which typically limits sample throughput to eight samples per day. This study evaluates a new approach using a novel laboratory mill and pulverizer to extract various pesticide residues from different kinds of plant materials and to identify and quantify the active ingredients by liquid chromatography tandem mass spectrometry (LC–MS–MS). It shows that by automating the procedure, the number of clean-up steps can be reduced significantly, which increases sample throughput over traditional approaches.
Cymoxanil (2-cyano-N-[(ethylamino) carbonyl]-2-(methoxyimino) acetamide) is the active ingredient in a number of pesticides manufactured by DuPont, for the control of various fungal diseases in crops and plants. It is mainly used for late blight potatoes, but is also applied to grapes, tomatoes, cucumbers and leafy vegetables. Its protection mechanism is to penetrate the surface to induce a host defence response to stop lesion growth and sporulation.
The typical pesticide residue set at DuPont contains eight samples and usually takes an eight-hour workday to complete. The goal of this study is to take advantage of recent developments in sample extraction with a new laboratory grinder-mill and improved instrument sensitivity and selectivity using liquid chromatography tandem mass spectrometry (LC–MS–MS) to increase the number of samples analysed per day by a factor of three. The methodology conforms to the US Environmental Protection Agency (EPA) Office of Prevention, Pesticides and Toxic Substances (OPPTS) Residue Chemistry Test Guidelines — OPPTS 860.1340 Residue Analytical Methods.1 These were developed by the EPA as guidance for developing analytical methods to be submitted to the agency for review under federal regulations and dictate that average recoveries must be 70–120% with good reproducibility.
Sample Preparation
Sample preparation for the analysis of pesticides and their residues has typically followed DuPont Report No. AMR 3705-95, "Analytical Method for the Determination of Famoxadone and Cymoxanil Residues in Various Matrices".2 In this procedure, ground-up samples are weighed into extraction jars, followed by the addition of water to allow them to rehydrate before extraction. Acetonitrile is added and samples are ground up with a laboratory homogenizer. The plant matrix is then allowed to settle-out and liquid extracts are filtered and collected in mixing cylinders containing sodium chloride. The mixing cylinders are capped, shaken and inverted to aid in the dissolution of sodium chloride and are then allowed to stand while the acetonitrile (upper layer) and water phases separate.
Traditional Pesticide Analysis Using Liquid Chromatography
For the analysis of cymoxanil, the acetonitrile aliquot is back extracted with hexane, concentrated, diluted with water and passed through a conditioned strong anion exchange solid-phase extraction (SPE) cartridge stacked on top of a conditioned carbon black SPE cartridge. Cymoxanil passes through the strong anion exchange cartridge and is retained on the carbon black cartridge. It is then selectively eluted off the cartridge and the concentrated cymoxanil residues are dissolved in a hexane–ethyl acetate mixture. The resulting solutions are passed through silica SPE cartridges. Cymoxanil is retained, selectively eluted and concentrated into a methanol–water mixture adjusted to pH 3. Samples are filtered and analysed by high performance liquid chromatography (HPLC) with UV detection.
Sample Preparation for Detection by LC–MS
There are approximately 20 clean-up steps in the AMR 3705-95 procedure, which limits the number of samples to 8–10 per day. A modification to this procedure, laid out in DuPont 13753, "Analytical Method for the Determination of Cymoxanil and its Metabolites in Leafy Vegetables Using LC–MS",3 significantly reduces the number of clean-up steps. In this procedure, samples are extracted using an acetonitrile–water mixture as described in DuPont Report No. AMR 3705 9, Revision 2. For cymoxanil, sodium chloride is added to an aliquot to separate the aqueous phase from the organic phase. The aqueous phase is discarded and the acetonitrile layer containing cymoxanil is passed through a strong anion exchange SPE column. The extract is then further cleaned up using a hexane liquid–liquid extraction followed by an Envi Carb (Supelco, Bellefonte, Pennsylvania, USA) SPE column. Cymoxanil is not retained on either of these columns, so the eluent is passed directly into an LC–MS system for analysis. Based upon the sensitivity and selectivity of LC–MS, the number of sample clean-up steps is reduced to approximately 10, allowing for the analysis of up to 16 samples per day.
Novel Approach to Sample Preparation
To further reduce the clean-up steps, a new sample preparation technique was evaluated that used a Geno/Grinder Model 2000 laboratory mill (Spex SamplePrep, Metuchen, New Jersey, USA). This is a laboratory mill or grinder specifically designed for vigorous vertical shaking of deep-well plates. It was originally designed to prepare plant tissue for extractions of nucleic acid, protein and other constituents by shaking the tissue, steel balls and a buffering agent together in each well of a titre plate. The application of this tool can be expanded to include micro-organisms when small silica beads are used instead of steel grinding balls. Microbes can also be disrupted in standard 96-well plates as opposed to deep well plates. Sample material that can be prepared includes bacteria, yeasts, molds, seeds, stems, roots, leaves and certain animal tissue. Because the vertical shaking motion of the equipment is so strong, many seeds and other forms of plant tissue can also be pulverized dry with the help of one or two grinding balls per well.
Sample Clean-up Steps
For the leaves of watery crops such as tomatoes and potatoes, 10 g of sample was extracted twice with 20 mL of a 90:10 mixture of acetonitrile–water solution. To ensure complete extraction of the compounds from the plant material, steel balls were added to the samples to pulverize the plant material. The samples were then extracted by shaking at high speed at approximately 700–1200 cycles/min, depending upon the sample. The sample extract was brought to 50 mL by adding acetonitrile. Approximately 1 mL of the extract was filtered using strong anion exchange and the SPE process described earlier. A 100 µL volume of sample was diluted to 1.0 mL in an HPLC vial with mobile phase and was analysed directly by LC–MS–MS. This approach cuts down the number of clean-up steps to three compared to 20 with the AMR 3705-95 method and 10 with the DuPont 13753 method.
Experimental
To evaluate the efficiency of the sample preparation and clean-up stage described previously, cymoxanil and its various metabolites were determined in a number of wet and dry crop and plant materials. All samples generated were analysed using LC–MS–MS.
This instrument is coupled with a 1100 Series HPLC system (Agilent Technologies, Santa Clara, California, USA) to first separate the biological species of interest, based upon their elution times off a column. The eluent is then introduced into the mass spectrometer for ionization to identify and quantify the species using triple-quadrupole MS. The principle of this technique is based upon confirmation of a particular molecular ion by the generation of its product species using collisionally induced dissociation or fragmentation.4 This technique, commonly known as multiple reaction monitoring (MRM), uses two resolving quadrupole mass filters, separated by another quadrupole, which is slightly pressurized by the introduction of a collision gas such as hydrogen or helium. In this MS–MS mode, the first quadrupole is used in a mass-resolving mode to select the precursor ion. The second quadrupole (or pressurized multipole collision cell) is used to produce fragmentation of the precursor ion. The final quadrupole is used in a mass-resolving mode to provide mass analysis of the resulting fragmented product ions. These species are then compared with reference spectra-data to produce unambiguous identification of the biomolecules of interest.

Table 1: Brief summary of the HPLC and LCâMSâMS instrumental conditions used for this study.
The benefit of the MS–MS design is that it also can be used in MS mode for quantification purposes. In this mode, the first quadrupole is typically used in the rf-only mode, as a wide bandpass filter to transmit molecular ions of a wide mass range. The collision cell is also used in the rf-only mode, but this time no collision gas is flowing, so the cell is just used to transmit ions to the last quadrupole, which is used in the mass resolving mode. This is known as the selective ion monitoring (SIM) mode. The ability to switch between both modes maximizes the amount of data being generated, particularly when small amounts of sample are being analysed. The higher number of product or fragmented ions identified leads to better confirmation of the parent molecules. This is especially important when analysing data from an LC separation. Cymoxanil, for example, which is an aliphatic nitrogen compound with a formula of C7H10N4O3 and molecular weight of 198, is typically eluted from the column after 10–15 min, over a period of 20–30 s. For this reason, it is very important to be able to switch very rapidly between SIM and MRM mode to collect both the molecular and fragmented information for positive and unambiguous identification of these species. After identification is made in the MS–MS mode, quantification of unknown samples can be performed in either the SIM or MRM modes.
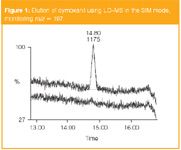
Figure 1
Instrument Methodology
Reversed-phase LC was used to separate cymoxanil and its metabolites from the rest of the extractants. The LC–MS–MS instrument was operated in single-ion monitoring MS negative-ion mode for quantitative analysis. Peak area was used for quantification. For confirmation of the presence of the analyte in unknown samples, the relative intensities of the fragmented ions were measured using the MRM mode. A brief summary of the HPLC and LC–MS–MS conditions are shown in Table 1.
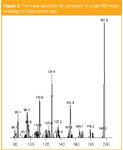
Figure 2
A 0.01 ppm cymoxanil LOQ fortification sample (following method DuPont 13753) and control (blank) in the SIM mode is shown in Figure 1. It can be seen very clearly that there is a significant peak eluted at about 15 min, which corresponds to the cymoxanil.
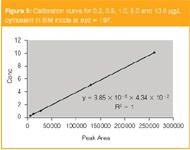
Figure 3
Confirmation of the compound is then made by measuring the intensities of the fragmented product ions in the MS–MS mode, by comparing them to known ratios in a database of pesticides. The mass spectrum (60–300 Da) of cymoxanil in single MS mode is shown in Figure 2, and the resulting calibration curve for 0.2, 0.5, 1.0, 5.0 and 10.0 µg/L in SIM mode (m/z = 197) is shown in Figure 3.

Table 2: Percentage recovery data and RSD for a 0.05 ppm (LOQ) and 0.50 ppm (10 x LOQ) reference samples run using three different analytical methods.
Table 2 shows some typical recovery data and RSD for 0.05 ppm and 0.50 ppm reference samples run using three different analytical methods.
Sample Preparation for Field-incurred Residues
Once the methodology was optimized for the separation and quantification of cymoxanil, the laboratory mill sample preparation and clean-up procedures were used for comparison purposes with traditional sample preparation methods. The basis of this method was then used to investigate an experimental new insecticide (DuP-1) and to identify and quantify its active ingredient and metabolites in various wet and dry plant and crop materials with field-incurred residues. Rather than fortifying directly onto a control sample in the laboratory, the crops were treated in the field with the insecticide at the timing and rate of typical agricultural practices. The crops were harvested at maturity and all detected metabolites have been incorporated into the plant matrix rather than on the surface, as is the case for laboratory fortifications. The results of this experiment will further test the extraction efficiency of the laboratory mill sample preparation procedure, which is shown in the following:
- Extract twice with acetonitrile–water in the grinder for 2 min at 1200 cycles/min.
- Dilute 1 mL of extract to 5 mL with water (adjust to pH 2.5).
- Filter through strong anion exchange SPE and collect the extract.
- Dilute the extract to 10.0 mL.
- Filter through a PTFE membrane into an LC vial.
- Analyse by LC–MS–MS.

Figure 4
Results
The total ion chromatogram (TIC) generated by the LC–MS–MS in MRM mode of the new experimental insecticide under investigation and its metabolites (A–G) is shown in Figure 4. Two pairs of metabolites — A and G, as well as D and E, are eluted from the LC column at the same time, indicating that they have similar chemical properties. A benefit of the LC–MS–MS method is that by monitoring the individual transitions of the MRM for each metabolite, the overlapping peaks can be resolved. Metabolite D with a mass of 444 Da can be identified by monitoring the transition 443 (molecular weight 1) to 298 Da and separated from metabolite E, which has a mass of 458 Da and a transition of 457 to 188 Da. Figure 5 shows the resulting MRM chromatograms for a 0.2 ppm standard of the metabolites D and E (top), together with a tomato sample spiked at the LOQ (bottom) and illustrates good resolution of metabolite D from E.

Figure 5
Table 3 compares the new sample preparation and clean-up method with a traditional homogenizer probe (Tekmar Tissumizer, Cincinnati, Ohio, USA) sample preparation using the DuPont-13753 method described earlier. It shows quantitative data (parts per million in sample) for the experimental new compound and one of its metabolites (D), for various crop samples. This methodology was then used to analyse a variety of wet, dry, oily and acidic crops for the active ingredient together with its metabolites. Table 4 shows spike recovery and RSD data for one of the watery crops (tomato), showing that the DuP-1 insecticide and all its metabolites are within the guidelines stated in EPA OPPTS 860.1340, which states that test data must have average recoveries of 70–120%. Although they are not presented here, the study also looked at other crops including limes (acidic crop), almonds (oily crop) and wheat straw (dry crop) and achieved similar spike recoveries and precision values.

Table 3: Comparison between the Geno/Grinder and Dupont-13753 sample prep methods for the quantitation of the experimental new compound (DuP-1) and one of its metabolites (D) in a variety of wet and dry crop samples.
Conclusion
It has been shown that the extraction of pesticide residues using a novel sample preparation procedure and identification and quantification of its active ingredients and metabolites using LC–MS–MS provides an extremely efficient, rugged and high-throughput analytical method. Compared with the traditional sample preparation, clean-up and detection methods, sample throughput was increased by a factor of three, from 8 samples to 24 samples per day. It also demonstrates that the extraction efficiency of the new procedure is valid for field-incurred residues in addition to laboratory-fortified samples.

Table 4
References
1. EPA, Office of Prevention, Pesticides and Toxic Substances (OPPTS) Residue Chemistry Test Guideline OPPTS 860.1340, Residue Analytical Methods.
2. DuPont Report No. AMR 3705-95: Analytical Method for the Determination of Famoxadone and Cymoxanil Residues in Various Matrices.
3. Dupont Report No. 13753: Analytical Method for the Determination of Cymoxanil and its Metabolites in Leafy Vegetables Using LC/MS.
4. B.A. Thomson et al., Anal. Chem., 67, 1696–1704 (1995).
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






