Novel Liquid Chromatography–Tandem Mass Spectrometry Method for Analysis of 102 Pesticides and 5 Mycotoxins Regulated by the State of Colorado in Dried Hemp
A novel liquid chromatography–tandem mass spectrometry (LC–MS/MS) method with a dual electrospray ionization (ESI) and atmospheric-pressure chemical ionization (APCI) source was developed for analyzing 102 pesticides and five mycotoxins that are regulated by the state of Colorado in hemp. The limit of quantitation (LOQ) of the 102 pesticides and 5 mycotoxins met Colorado state action limit requirements for these analytes in a hemp matrix. For this study, 88 out of 102 pesticides and all 5 mycotoxins were analyzed using LC–MS/MS with an ESI source, whereas the remaining 14 pesticides were determined using LC–MS/MS with an APCI source. A simple, fast, green, and cheap acetonitrile solvent extraction method was used to extract the pesticides and mycotoxins from the hemp matrix with good extraction efficiency in the range of 80–120%. A hemp matrix is challenging and causes matrix effects such as ion suppression or enhancement. The LC method was optimized and 30 internal standards were added to reduce and compensate for these matrix effects to obtain method accuracy in the range of 70–120%. The ionization mechanism of nonpolar pesticides (normally analyzed by gas chromatography–tandem mass spectrometry [GC–MS/MS]) with an APCI ion source was elucidated.
The Agriculture Improvement Act of 2018 (2018 Farm Bill) authorized the production of hemp, removing hemp and hemp seeds from the Drug Enforcement Administration’s (DEA) schedule of Controlled Substances if they contain less than 0.3% tetrahydrocannabinol (THC), which gives users a high (1). Like any other agricultural crop, pesticides are applied to hemp plants to protect them from pests and improve growth yield. Chronic exposure to pesticides can lead to serious health risks; therefore, pesticide analysis in hemp is important for consumer safety and quality control. Because there are no federal regulations for pesticide analysis in hemp, individual states define and regulate the use of pesticides for the production of hemp. Among these states, Oregon, California, and Florida have set action levels for 59, 66, and 67 pesticides, respectively, in cannabis and hemp (2–4). Outside the United States, Canada has set maximum residue limits (MRLs) for 96 pesticides in cannabis-based products (5). Recently, Colorado state has issued action limits for 102 pesticides (including all found on Canada list, and 6 more) in hemp (6). In addition to pesticide residues, Canada, Colorado, and other U.S. states also require hemp and cannabis products to be tested for five mycotoxins (four aflatoxins and ochratoxin A) (3–6). Mycotoxin contamination can occur during the cultivation or storage of hemp. Like pesticides, these mycotoxins are toxic and pose a serious health risk to consumers. As a result, testing for the levels of pesticide and mycotoxins in hemp is important to ensure the health of consumers and quality control in the United States and Canada.
In the past, pesticide analysis was performed using both liquid chromatography–tandem mass spectrometry (LC–MS/MS) and gas chromatography–tandem mass spectrometry (GC–MS/MS) methods, coupled with sample preparation methods such as QuEChERS (quick, easy, cheap, effective, rugged, and safe) and solvent extraction with either dispersive solid-phase extraction (dSPE) or solid-phase extraction (SPE) cleanup. These reported methods are time-consuming, expensive, slow, less environmentally friendly, and show poor extraction efficiency for a few analytes such as daminozide and others (7–10). In the past, we published our novel LC–MS/MS method to meet regulations for pesticides and mycotoxins regulated by different U.S. states and Canada in different cannabis matrices (11–15). The aim of this study was to validate a novel, cost-effective, and fast LC–MS/MS method to analyze all 102 pesticides and 5 mycotoxins regulated by Colorado state in hemp matrix. The method uses a simple solvent extraction with extraction efficiency in the range of 80–120% and LC–MS/MS method with a dual electrospray ionization (ESI) and atmospheric-pressure chemical ionization (APCI) source to meet action limits set by Colorado state for 102 pesticides and 5 mycotoxins in hemp matrix (6). Hemp is a challenging matrix for analysis of pesticides and mycotoxins because of its complex chemical composition. Both LC and MS methods were optimized to reduce matrix effects, thereby improving method sensitivity, and 30 internal standards were utilized to compensate for matrix effects to achieve method accuracy in the range of 70–120%.
Experimental
Materials and Reagents:
Pesticide and mycotoxin standards were purchased from Accustandard and Sigma-Aldrich, respectively. To compensate for matrix effects observed in complex hemp matrices, 30 internal standards solution were used from the PerkinElmer One Pesticide ISO17034 CRM Reagent Kit to improve method recovery and quantitation accuracy.
Hardware/Software:
Chromatographic separation was conducted on a PerkinElmer LX50 UHPLC system, while detection was achieved using a PerkinElmer QSight 420 MS/MS detector with a dual ionization ESI and APCI source, which operated independently with two separate inlets. All instrument control, data acquisition, and data processing was performed using the Simplicity 3Q software platform.
Sample Preparation Method:
Below is the step-by-step sample preparation procedure with a 10-fold dilution.
- Take approximately 5 g of dried hemp as representative of each sample batch, and grind it finely using a grinder (Omni Bead Ruptor 96).
- Measure 1 g of sample, and place it into a 50 mL centrifuge tube.
- Add 5 mL of LC–MS-grade acetonitrile to the tube and cap it.
- Place the tube on multi-tube vortex mixer, and allow it to vortex for 10 min.
- Centrifuge extract in tube for 10 min at 3000 rpm.
- Filter the solvent into a 5 mL glass amber vial using a 0.22 micrometre nylon syringe-filter and cap it.
- Label the bottle with the sample ID.
- Transfer 0.5 mL of extracted sample into a 2 mL HPLC vial, dilute it with 0.49 mL of LC–MS-grade acetonitrile, and mix it. Spike 10 μL of 30 internal standards solution from a PerkinElmer One Pesticide ISO17034 CRM Reagent Kit.
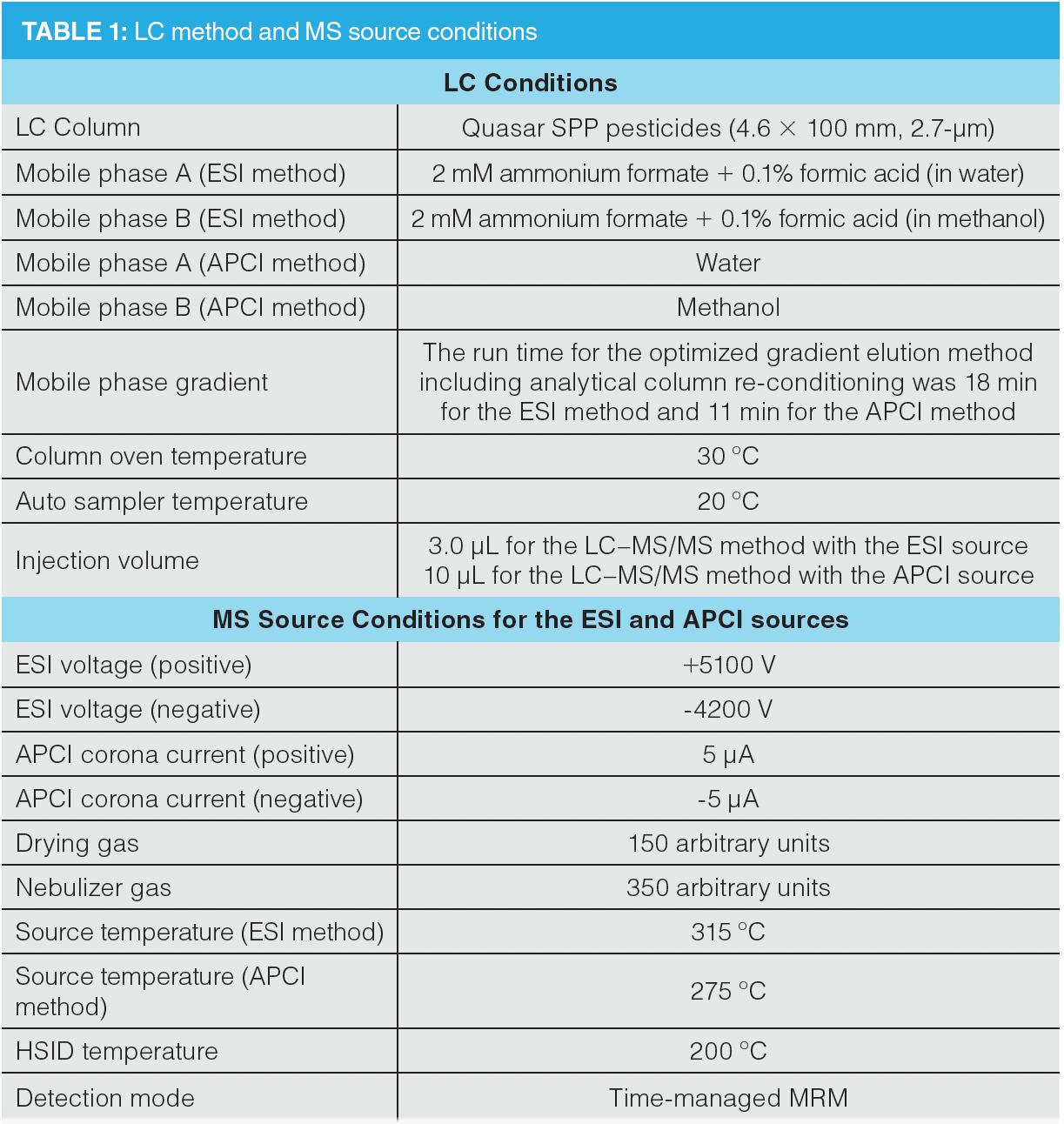
LC Method and MS Source Conditions:
The LC method and MS source parameters are shown in Table 1.
Results and Discussion
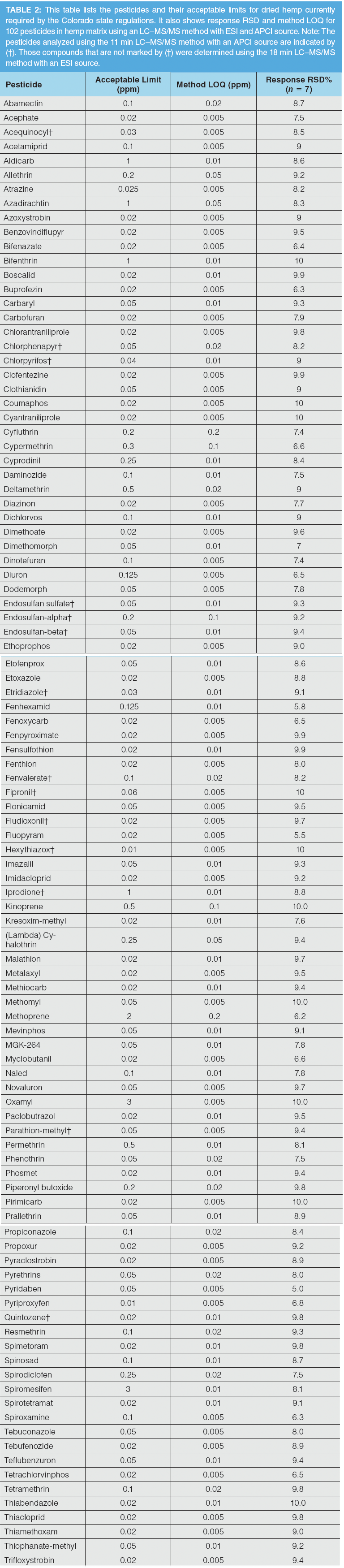
Detection Limits:
The detection limit for each pesticide and mycotoxin was determined by pre-spiking dried hemp matrix with different low levels and measuring signal to noise and response reproducibility at each level with the LC–MS/MS method using either the ESI or APCI source. The limit of quantitation (LOQ) for each pesticide and mycotoxin was determined to be the lowest concentration for pre-spiked hemp matrix, at which S/N = 10 or higher and the response relative standard deviation (RSD) (n = 7) less than 15% was measured for quantifier ions. Colorado’s acceptable limits, method LOQs, and response RSD for each of the pesticides and mycotoxins in dried hemp are summarized in Tables 2 and 3, respectively. The LC–MS/MS method with dual ESI and APCI sources analyzes the 102 pesticides and 5 mycotoxins in dried hemp, as required by Colorado regulations with 2 separate injections using the same instrument platform with a total run time of 29 min. A diverter valve enables quick and automated switching of mobile phase eluent to the APCI source from the ESI source and vice versa to facilitate the fast analysis using this method without requiring the time-consuming step of switching APCI and ESI ion sources in LC–MS/MS systems with a single source. A total of 88 out of 102 pesticides and 5 mycotoxins were analyzed using the ESI source, and the remaining 14 pesticides regulated by the State of Colorado were measured with the APCI source. As demonstrated in Tables 2 and 3, the LOQs determined in this study met the Colorado action limits for all the analyzed pesticides and mycotoxins in the hemp matrix with a single LC–MS/MS instrument platform to reduce cost and complexity of analysis. The LOQ of approximately 83% and 94% of analytes in dried hemp was less than or equal to 0.01 and 0.02 ppm, respectively.
Sample Preparation Extraction Efficiency:
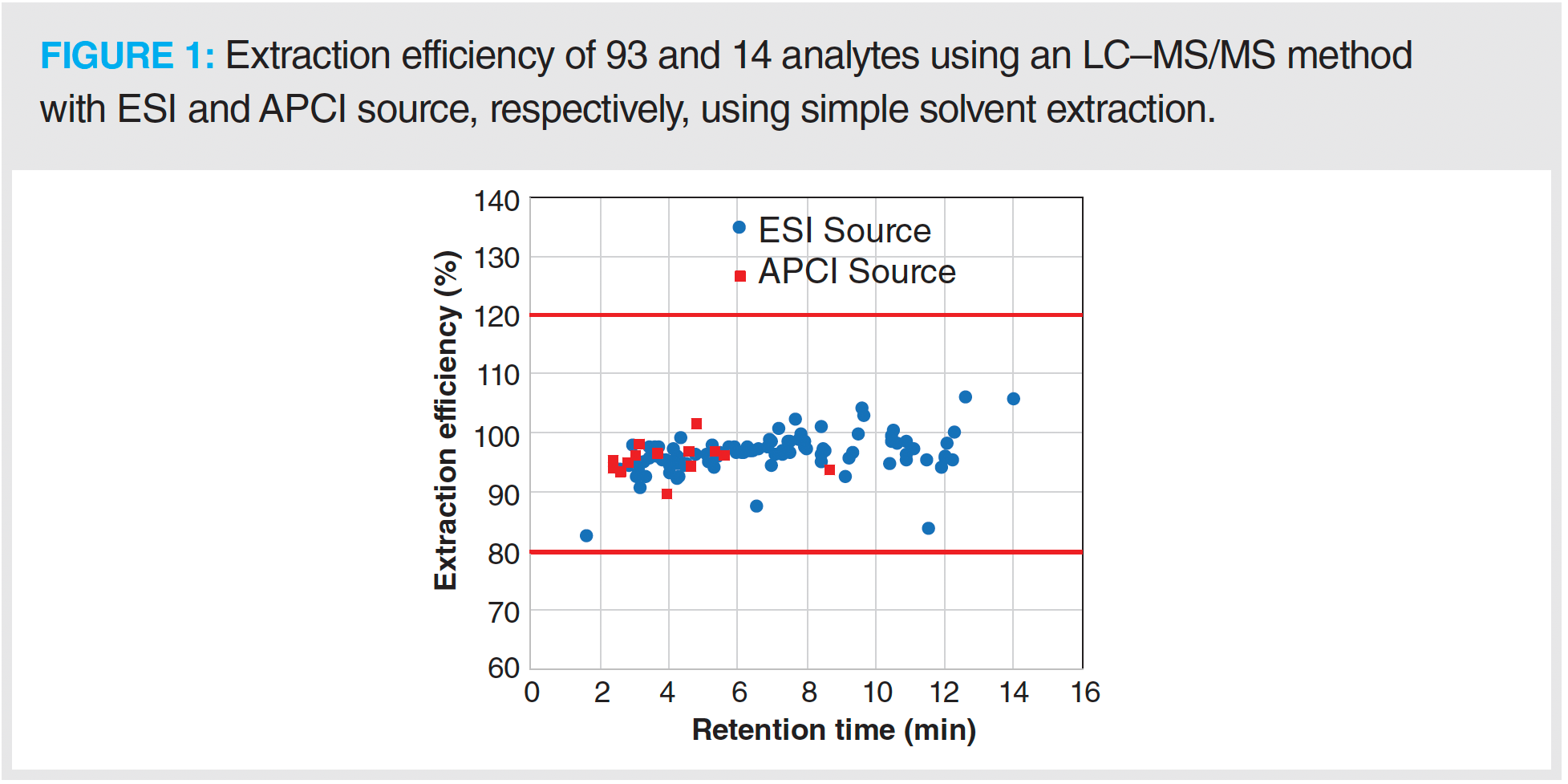
Different groups have developed complex sample preparation methods using either solvent extraction or QuEChERS extraction followed by either SPE or dSPE with different sorbents (7–10). A number of analytes (such as daminozide, imazalil, spirotetramat, pyridaben, and a few others) showed poor extraction efficiency with such intricate, expensive, and time-consuming sample preparation methods. Because daminozide is too polar to be extracted efficiently with QuEChERS, it remains in the aqueous phase and does not partition into the organic solvent during the salting-out step. The recovery of daminozide from a cannabis matrix with QuEChERS extraction has been reported to be less than 10% (9). The extraction efficiency of other pesticides, such as imazalil, spirotetramat, pyridaben, and a few others, was lower than the ideal value of 80% with sample preparation methods using dSPE or SPE cleanup because these pesticides are retained on sorbents used in these sample preparation methods (10). Recently, another group developed a solvent extraction method followed by an evaporation step to carry out solvent exchange with initial LC mobile phase for extraction of pesticides from a hemp matrix. The extra evaporation step in this sample preparation method resulted in low extraction efficiency or recoveries for some of the pesticides such as abamectin, fenpyroximate, pyridaben, and others because of their precipitation (16). As a result of the low extraction efficiency of some of the pesticides with time-consuming, expensive, and difficult sample preparation methods such as solvent extraction with evaporation, QuEChERS, or solvent extraction followed by dSPE or SPE cleanup to extract pesticides and mycotoxins from cannabis and hemp matrix, we used a simple acetonitrile-based solvent extraction method to get good extraction efficiency with high throughput. The fortified dried hemp samples were used to determine pesticides and mycotoxins extraction efficiency. Seven dried hemp samples were spiked at two levels (low and high) of the 102 pesticides (0.02 and 0.2 ppm) and 5 mycotoxins (4 and 40 ppb) standard. The extraction efficiency of all of the 102 pesticides and 5 mycotoxins at 2 different levels was within an acceptable range of 80–120%, with RSD less than 20% for 7 dried hemp samples. Figure 1 shows the extraction efficiency of 93 analytes (88 pesticides and 5 mycotoxins) and 14 pesticides at high level with ESI and APCI source method, respectively. Note that the extraction efficiency for few pesticides at low level was not determined because their LOQ was higher than 0.02 ppm in hemp matrix. The sample preparation extraction efficiency was calculated by taking the ratio of signal of analyte in pre-spiked to post-spiked extract and multiplying this number by 100.
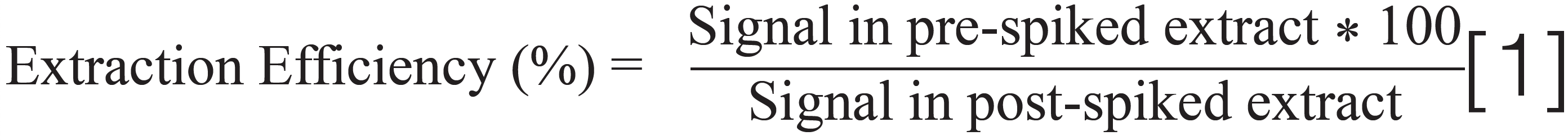
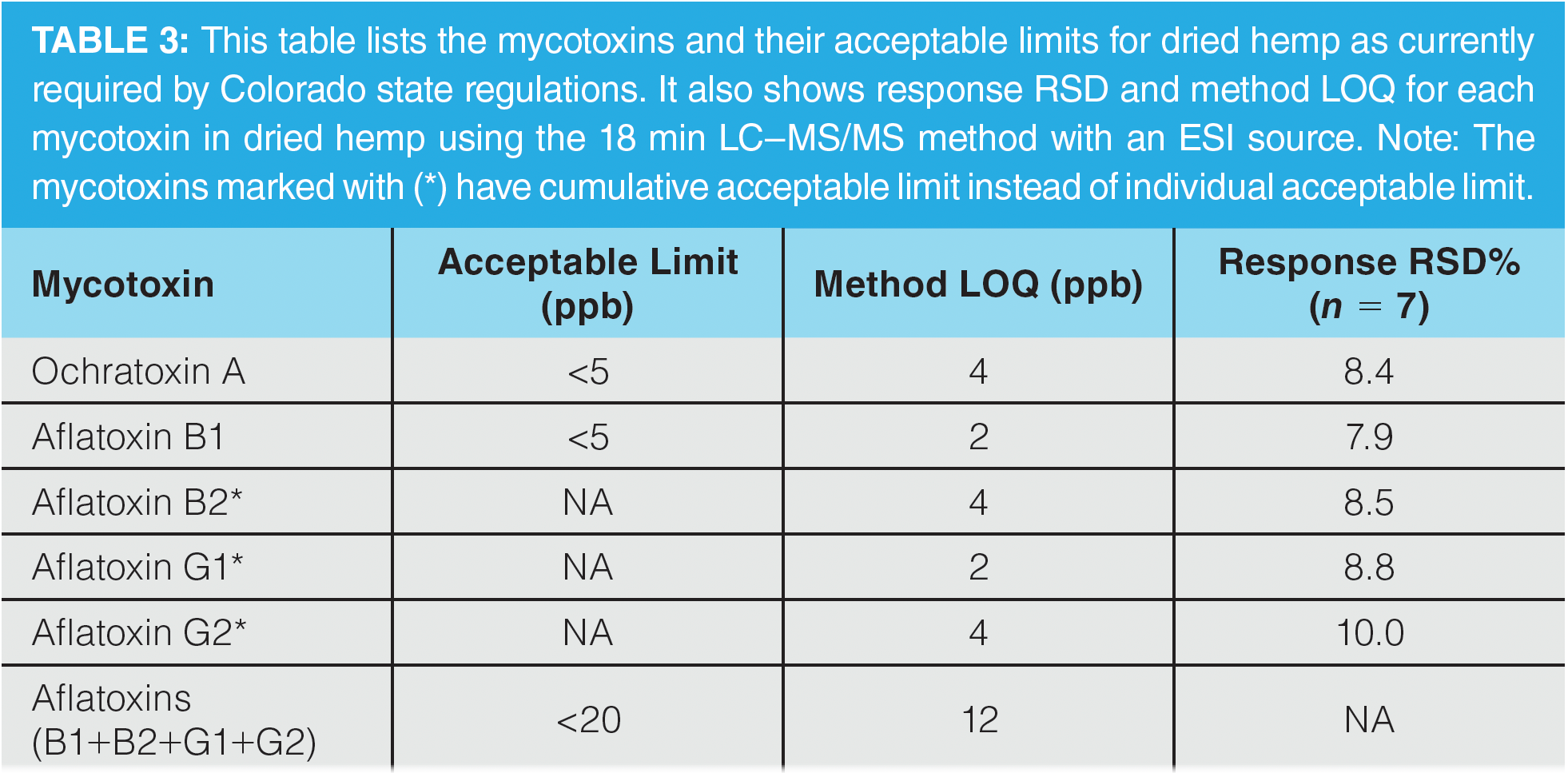
Matrix Effects and Method Accuracy:
Hemp is a challenging matrix and causes matrix effects, such as ion suppression and enhancement, because many of the highly concentrated components (such as cannabinoids and terpenes) are also co-extracted with pesticides and mycotoxins. The ion suppression and enhancement effects were determined by checking the signal for spiked pesticides and mycotoxins at a fixed concentration in a dried hemp matrix extract and solvent standard. The matrix effect calculation was performed by taking the percentage difference between the signal of the analyte in the post-spiked hemp extract and clean solvent and dividing it by the signal in clean solvent. About one-third of analytes exhibited absolute matrix effects greater than 20% with the LC–MS/MS method. Because hemp matrix is very hydrophobic, absolute matrix effects greater than 20% were mainly observed at higher elution time for analytes with a reversed-phase C18 LC column. Moreover, matrix effects are different in various forms of hemp and cannabis products, such as different types of cannabis and hemp plants and concentrates with different cannabinoids, terpene profiles, and edible products (tinctures, gummies, and chocolates) with high sugar and fat content. Therefore, it is difficult to determine a universal matrix that can represent different forms of cannabis and hemp products for carrying out single matrix matched calibration for accurate quantitation of pesticides and mycotoxins in different types of cannabis and hemp products. The matrix-matched calibration using different strains of cannabis and hemp flower, types of concentrate, and edible products for quantitation of pesticides and mycotoxins in different cannabis and hemp products is not a practical option to achieve high throughput at cannabis testing laboratories because of its complexity and slow cycle time. The practical approach is to use calibration with solvent standards for quantitation of pesticides and mycotoxins in different cannabis and hemp products to improve throughput with less complexity. In this case of solvent calibration, it is vital to add internal standards to both solvent standard calibrants and hemp samples because they would improve method accuracy by compensating for sample matrix effects, such as ion suppression and enhancement effects from hemp matrix. A solution containing 30 internal standards was spiked in both solvent standards used for generation of calibration curves for quantitation and hemp samples to improve the accuracy of quantitative analysis by compensating for the matrix effects and correcting for any inaccuracies in sample injection in LC. The method accuracy of the LC–MS/MS method is given by the following equation:
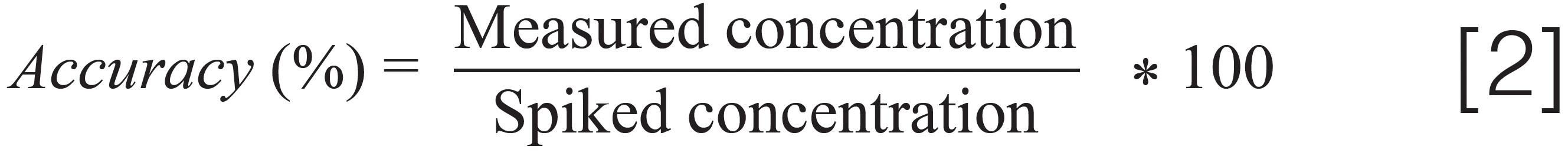
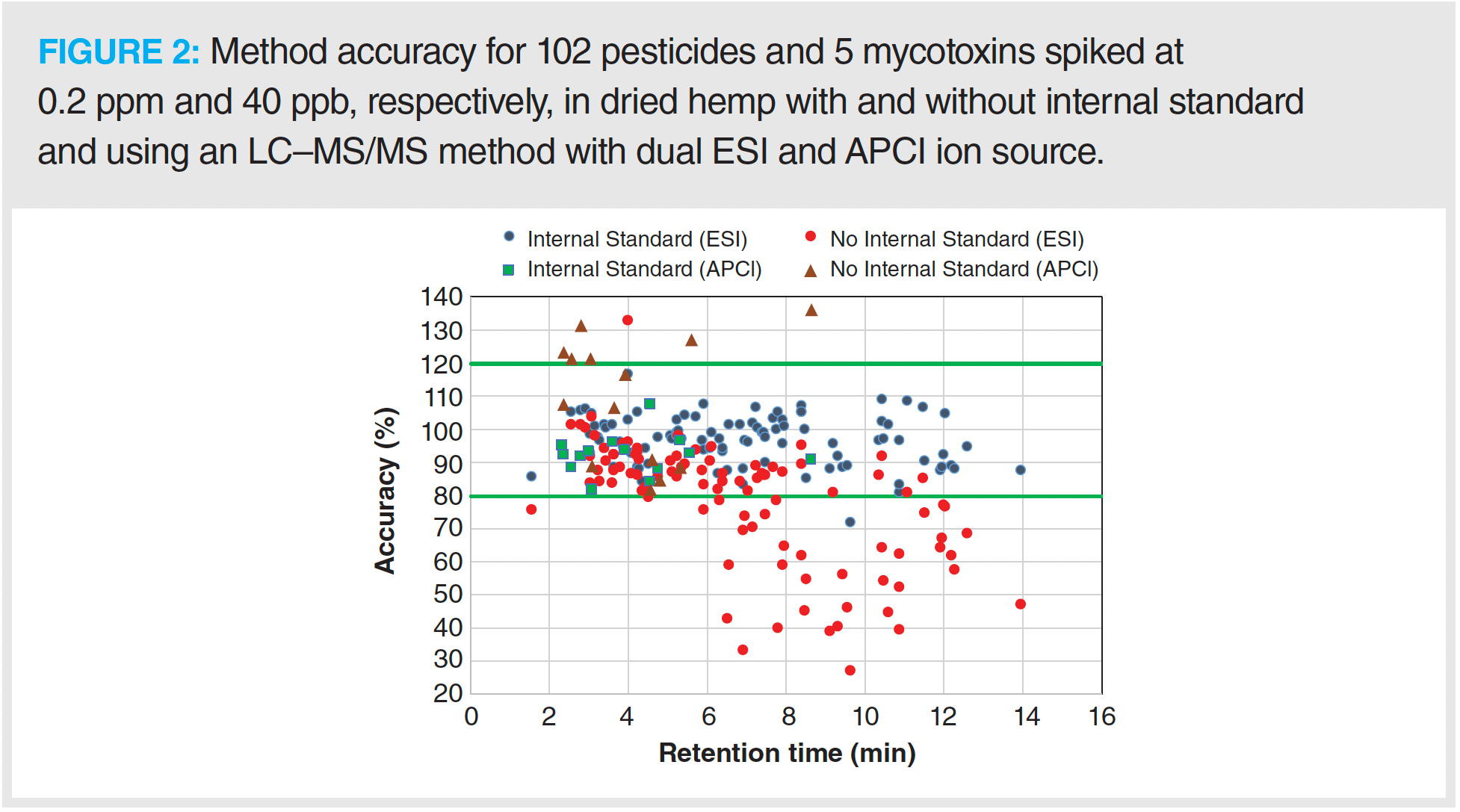
A total of 7 dried hemp samples were spiked at 2 levels (low and high) of the 102 pesticides (0.02 and 0.2 ppm) and 5 mycotoxins (4 and 40 ppb) standard. The method accuracy of all 102 pesticides and 5 mycotoxins at 2 different levels were within an acceptable range of 70–120%, with RSD less than 20% for 7 dried hemp samples with internal standards. Figure 2 shows the method accuracy of 93 analytes (88 pesticides and 5 mycotoxins) and 14 pesticides at high level with ESI and APCI source method, respectively, with and without internal standards. Note that method accuracy for a few pesticides at the low level was not determined because their LOQ was higher than 0.02 ppm in hemp matrix. The data illustrate that adding internal standards resulted in 70–120% accuracy for 102 pesticides and 5 mycotoxins with the LC–MS/MS method. Aside from 1 pesticide, the method accuracy for 101 pesticides and 5 mycotoxins was in the range of 80–120%.
Method Optimization to Reduce Matrix Interference:
Hemp is a difficult matrix because it shows substantial matrix interference, caused by the presence of isobaric compounds, for the signalling of some pesticides. To improve the selectivity of pesticide analysis in hemp, it is necessary to have multiple mass transitions (more than two) for a few compounds to find a mass transition that does not have matrix interference. For example, propiconazole shows the highest signal based on protonated molecular ion transition in a standard, but the multiple reaction monitoring (MRM) transition (342.1 to 69), based on protonated molecular ion in the hemp matrix, showed poor LOQ of 0.5 ppm because of matrix interference from co-extracted compounds isobaric to this pesticide in hemp matrix. Therefore, MRM transition (344.1 to 69) based on the M+2 isotope mass of the protonated molecular ion of propiconazole was determined to reduce matrix interference and achieve a LOQ of 0.02 ppm for propiconazole in the hemp matrix. Similarly, we had to determine optimum MRM transitions for other pesticides such as acequinocyl, prallethrin, pyrethrins, and others to reduce matrix interference.
Analysis and Ionization Mechanism for Nonpolar Analytes Using an APCI Source:
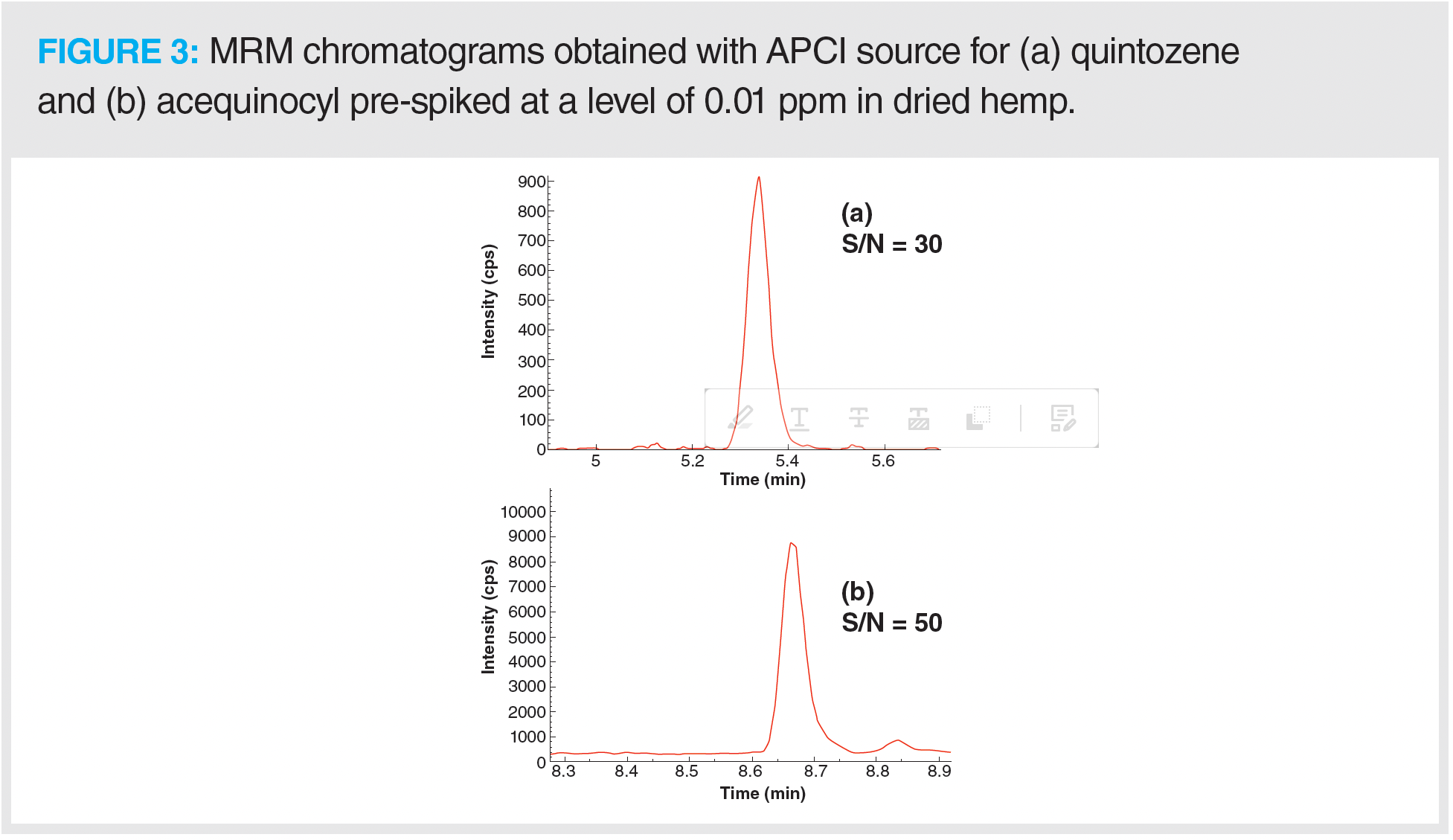
A number of hydrophobic and nonpolar pesticides (quintozene, chlorfenapyr, methyl parathion, iprodione, fenvalerate, and others) found on the Colorado list are traditionally analyzed by GC–MS/MS because they do not ionize effectively when analyzed using LC–MS/MS with an ESI source. These analytes either have low proton affinity or lack acidic functional groups and are difficult to ionize by an ESI source in either positive or negative ion mode. An APCI ion source is much better suited for ionization of these hydrophobic and nonpolar analytes, and it was used to determine the detection limits of these hydrophobic analytes in hemp. Using an 11 min LC–MS/MS method with an APCI source, the LOQs of 14 nonpolar pesticides in dried hemp were in the range of 0.005–0.1 ppm. Figures 3(a) and 3(b) show an excellent S/N ratio for two representative pesticides (quintozene and acequinocyl) pre-spiked at a level of 0.01 ppm in the dried hemp matrix using an LC–MS/MS method with an APCI source. This demonstrates the extreme sensitivity of the APCI method. Most analytes with basic or acidic functional groups are ionized in ESI and APCI source with either protonation in positive ion mode or deprotonation in negative ion mode. As some of the analytes analyzed using the APCI method could not be ionized with the above mentioned ionization (protonation/deprotonation) mechanism, we had to determine other ways of ionizing these compounds using APCI ion source. For ionization of compounds in APCI source, different ionization mechanisms such as proton attachment, proton abstraction, anion adduction, electron capture, and dissociative electron capture have been proposed in the past (17). It was demonstrated that chlorinated nitrobenzene compounds can form phenoxide ions under negative APCI conditions (18). Similarly, we proposed the mechanism for ionization of quintozene with APCI source in negative ion mode (12,13,15). The different ionization mechanisms of pesticides analyzed in hemp matrix with APCI ion source were determined by collecting first quad scan mass spectra over a range of 50–500 Da of different analytes. These are presented below:
Proton attachment – chlorpyrifos, hexythiazox
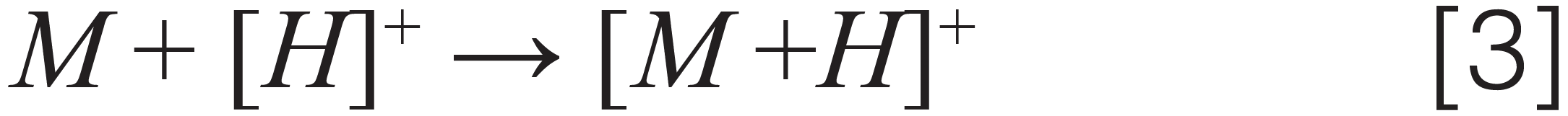
Proton abstraction – fipronil, fludioxinil, endosulfan-alpha, endosulfan-beta, and endosulfan sulfate
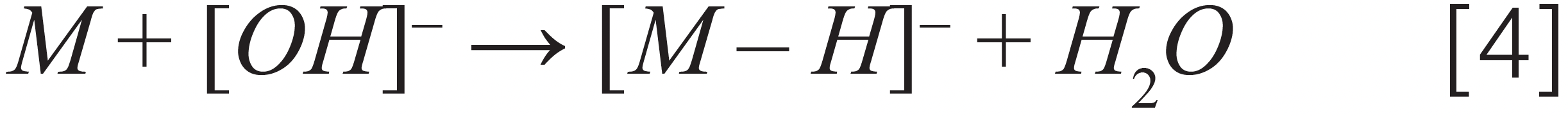
Anion attachment – chlordane
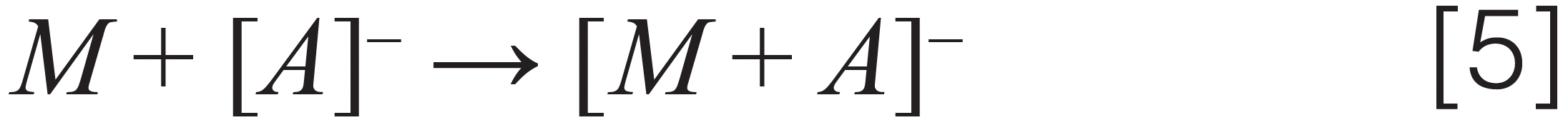
Electron capture – acequinocyl
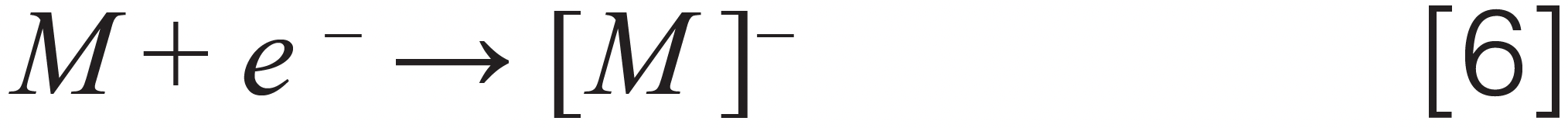
Dissociative electron capture – chlorfenapyr, parathion-methyl, etridiazole, fenvalerate, iprodione, and captan
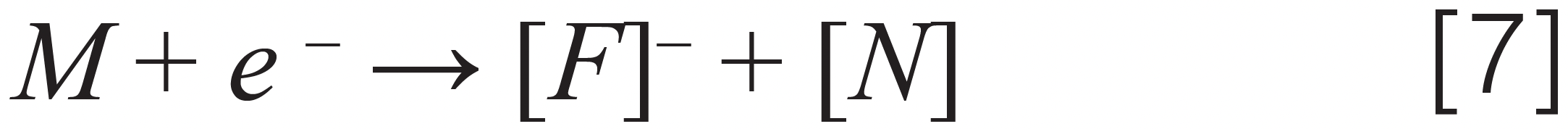
Electron capture by oxygen followed by chemical reaction – quintozene
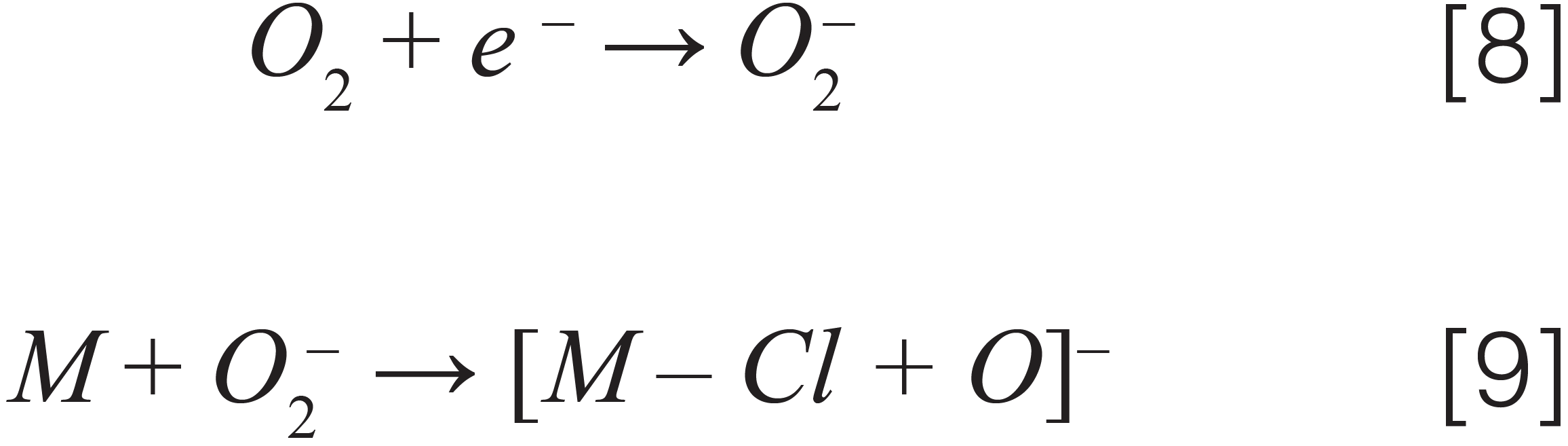
Where [M] stands for different pesticides, [A]– stands for an anion, [F]– stands for a fragment ion, and [N] stands for a neutral fragment. Note that we demonstrated the ionization mechanism for two more pesticides (captan and chlordane), not on Colorado’s list but present in cannabis regulations for other U.S states, such as California and Florida.
Determination of Nonpolar Pesticides with ESI Source
A number of nonpolar pesticides do not have low detection limits using LC–MS/MS systems with a conventional ESI source. Because we are using a heated sheath of gas around the electrospray source plume or droplets, we were able to ionize these compounds with good sensitivity in dried hemp. The detection limits of these nonpolar pesticides (allethrin, bifenthrin, cyfluthrin, cypermethrin, deltamethrin, kinoprene, lambda-cyhalothrin, methoprene, MGK-264, permethrin, phenothrin, resmethrin, tetramethrin, and a few others) were in the range of 0.01–0.2 ppm in dried hemp to meet Colorado action limits in hemp.
Conclusions
In this study, a novel LC–MS/MS method with a dual ESI and APCI source using simple solvent extraction for sample preparation was validated for analyzing 102 pesticides and 5 mycotoxins regulated by Colorado state in dried hemp. This method enabled the identification and quantification of all analytes, with the LOQ in the range of 0.002 to 0.2 ppm, which meets the action limits set by the state of Colorado in dried hemp matrix. Unlike other published sample preparation methods, simple solvent extraction showed excellent extraction efficiency in the range of 80–120%, with RSD less than 20% for all 102 pesticides and 5 mycotoxins. Hemp matrix is challenging, and causes matrix effects and interference. We minimized matrix interference by optimizing the LC–MS/MS method and added 30 internal standards to our method to compensate for matrix effects; this helped us in attaining method accuracy in the range of 70–120%, with RSD less than 20%, for all 102 pesticides and 5 mycotoxins. The method demonstrated good robustness, precision, high throughput, and reproducibility. The different ionization mechanisms of nonpolar pesticides with APCI ion source was elucidated. The LC–MS/MS method worked well for a wide range of polar and nonpolar analytes, including the analytes normally analyzed by GC–MS/MS. The ability to screen and quantitate all 102 pesticides and 5 mycotoxins, including the compounds analyzed on a GC–MS/MS system, makes this LC–MS/MS method a novel way to screen and quantitate pesticides and mycotoxins in dried hemp with a single instrument platform.
References
(1) The Farm Bill, Hemp Legalization and the Status of CBD: An Explainer. https://www.brookings.edu/blog/fixgov/2018/12/14/the-farm-bill-hemp-and-cbd-explainer/ (accessed 2022-07-27).
(2) Oregon Administrative Rules 333–007–0400 Pesticide Analytes and Their Action Levels. https://www.oregon.gov/oha/PH/DISEASESCONDITIONS/CHRONICDISEASE/MEDICALMARIJUANAPROGRAM/Documents/rules/333-007-0400_0410_0415_0425_Exhibit_A_eff_March312022.pdf (accessed 2022-07-27).
(3) California Code of Regulations, Title 16, Division 42, Bureau of Cannabis control, Article 5, § 5719. https://bcc.ca.gov/law_regs/readopt_text_final.pdf (accessed 2022-07-27).
(4) Florida State Regulations for Pesticide and Mycotoxin Analysis in Cannabis Flower. https://www.flrules.org/gateway/ruleNo.asp?id=64ER20-9 (accessed 2022-07-27).
(5) Health Canada, Mandatory Cannabis Testing for Pesticide Active Ingredients—List and Limits. https://www.canada.ca/en/public-health/services/publications/drugs-health-products/cannabis-testing-pesticide-list-limits.html (accessed 2022-07-27).
(6) Colorado Department of Public Health and Environment Regulations for Colorado Wholesale Food, Industrial Hemp, and Shellfish 6 CCR 1010–21 https://drive.google.com/file/d/15kEVNcFFdP07OFaalZ8D5OG4KEStUxce/view (accessed 2022-07-27).
(7) Stenerson, K. K.; Oden, G. Improved Workflow in the Analysis of Pesticide Residues in Cannabis by GC–MS/MS and LC–MS/MS. Cann. Sci. & Tech. 2018, 1 (1), 48–53.
(8) Kowlaski, J.; Dahl, J. H.; Rigdon, A.; et al. Evaluation of Modified QuECHERS for Pesticide Analysis in Cannabis. LCGC North Am. 2017, 35 (5s), 8–22.
(9) Wang, X.; Mackowsky, D.; Searfoss, J.; Telepchak, M. Novel LC–MS/MS Method with a Dual ESI and APCI Ion Source for Analysis of California-Regulated Pesticides and Mycotoxins in Medium-Chain Triglyceride (MCT) Oil Cannabis Tinctures. LCGC North Am. 2016, 34 (10), 20–27.
(10) Moulins, J. R.; Blais, M.; Montsion, K.; et al. Multiresidue Method of Analysis of Pesticides in Medical Cannabis. J. AOAC Int. 2018, 101 (6), 1948–1960. DOI: 10.5740/jaoacint.17-0495
(11) Dalmia, A.; Cudjoe, E.; Astill, T.; et al. LC–MS/MS with ESI and APCI Sources for Meeting California Cannabis Pesticide and Mycotoxin Residue Regulatory Requirements. Cann. Sci. & Tech. 2018, 1 (3), 38–50.
(12) Dalmia, A. Systems and Methods for Pesticide Detection Using Mass Spectroscopy. US 10914713, 2021.
(13) Dalmia, A.; Johnson, C.; Hariri, S.; et al. Novel LC–MS/MS Method with a Dual ESI and APCI Ion Source for Analysis of California-Regulated Pesticides and Mycotoxins in Medium-Chain Triglyceride (MCT) Oil Cannabis Tinctures. Curr. Trends Mass Spec. 2020, 18 (3), 22–29.
(14) Dalmia, A.; Cudjoe, E.; Jalali, J.; et al. A Novel ESI and APCI LC/MS/MS Analytical Method for Meeting the Canadian Cannabis Pesticide Residues Regulatory Requirements. 2019. https://resources.perkinelmer.com/lab-solutions/resources/docs/app_69954-cannabisconcentrates_final.pdf (accessed 2022-07-27).
(15) Dalmia, A.; Cudjoe, E.; Jalali, J.; Qin, F. A LC–MS/MS Method with Electrospray Ionization and Atmospheric Pressure Chemical Ionization Source for Analysis of Pesticides in Hemp. J. Cannabis Res. 2021, 3, 50. DOI: 10.1186/s42238-021-00106-9
(16) Michlig, N.; Lehotay, S. J.; Lightfield, A. R.; Beldoménico, H.; Repetti, M. R. Validation of a High-Throughput Method for Analysis of Pesticide Residues in Hemp and Hemp Products. J. Chromatography A 2021, 1645, 462097. DOI: 10.1016/j.chroma.2021.462097
(17) McEwen, C. N.; Larsen, B. S. Ionization Mechanisms Related to Negative Ion APPI, APCI, and DART. J. Am. Soc. Mass. Spectrometry 2009, 20, 1518–1521. DOI: 10.1016/j.jasms.2009.04.010
(18) Carroll, D. I.; Dzidic, I.; Stillwell, R. N.; Horning, M. G.; Horning, E. C. Subpicogram Detection System for Gas Phase Analysis Based Upon Atmospheric Pressure Ionization (API) Mass Spectrometry. Anal. Chem. 1975, 47 (8), 1308–1312. DOI: 10.1021/ac60342a009
Avinash Dalmia is a senior principal application scientist with PerkinElmer, in Shelton, Connecticut, USA.

Reversed-Phases for LC Deliberately Doped with Positive Charge: Tips and Tricks for Effective Use
May 13th 2025In this month's edition of LC Troubleshooting, Dwight Stoll and his fellow researchers discuss both the benefits (improved peak shape/loading) and challenges (excessive interaction) associated with charge-doped reversed-phase (RP) columns for both analytical and preparative separations.
Evaluating the Accuracy of Mass Spectrometry Spectral Databases
May 12th 2025Mass spectrometry (MS) can be effective in identifying unknown compounds, though this can be complicated if spectra is outside of known databases. Researchers aimed to test MS databases using electron–ionization (EI)–MS.
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








