Market Profile: High-Content Screening
High-content screening (HCS) is a high-throughput method that enables the functional analysis of cells. HCS plays a vital role for drug discovery applications, including drug targets verification and pathway detection, in both the pharmaceutical and biotechnology industries.
High-content screening (HCS) is a high-throughput method that enables the functional analysis of cells. HCS plays a vital role for drug discovery applications, including drug targets verification and pathway detection, in both the pharmaceutical and biotechnology industries. Often the technology is used in neuroscience research, especially in drug development for Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative diseases.
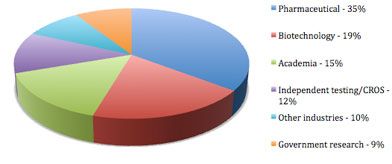
High-content screening industry distribution for 2011
The first step of performing an HCS experiment is to label the cell of interest with a fluorescence biomarker. After the cells are loaded into the wells and placed into the instrument, a particular wavelength of light is shined onto the cells, which illuminates the labeled cells and gives the embedded camera the opportunity to take photos.
One drawback of HCS is the difficulty of analyzing the vast amount of data obtained. Both HCS instrument manufacturers and third-party software vendors have been allocating significant resources into developing more accommodative software to address this need. Another obstacle of HCS is its lackluster image resolution. However, the development of higher resolution digital cameras, faster shutters, automated microscopes, quantum dots, and new fluorophores has dramatically improved the resolution.
Pharmaceutical, biotechnology, academia, CRO, and government research industries combined account for 90% of the global high-content screening demand. Other application areas include agriculture and food, hospitals and clinical laboratories, government testing, and environmental laboratories.
The foregoing data were extracted and adapted from SDi's A Complete Life Science Perspective report. For more information, contact Glenn Cudiamat, VP of Research Services, Strategic Directions International, Inc., 6242 Westchester Parkway, Suite 100, Los Angeles, CA 90045, (310) 641-4982, fax: (310) 641-8851, e-mail: cudiamat@strategic-directions.com
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Top Execs from Agilent, Waters, and Bruker Take the Stage at J.P. Morgan Healthcare Conference
January 16th 2025The 43rd Annual Healthcare J.P. Morgan Healthcare Conference kicked off in San Francisco earlier this week. Here’s what top executives from Agilent, Bruker, and Waters, discussed during the event.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

