Market Profile: Dissolution Testing
Dissolution testing is a mandatory test for the physical evaluation of solid dosage forms, such as capsules, tablets, ointments, and creams. The most basic form of testing measures the rate of dissolution or solubility of a drug tablet.
Dissolution testing is a mandatory test for the physical evaluation of solid dosage forms, such as capsules, tablets, ointments, and creams. The most basic form of testing measures the rate of dissolution or solubility of a drug tablet. Dissolution testing can also be used in absorption, distribution, metabolism, and excretion (ADME) and bioavailability studies, to test release rates of a drug substance under different conditions, and to provide information about the efficacy of in vivo performance.
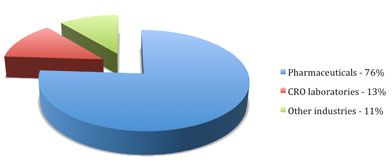
2011 dissolution testing demand by industry
It is a common practice to link a dissolution tester with a high performance liquid chromatography (HPLC) system. This perhaps is one of the fastest growing areas in dissolution testing market. When the samples are introduced into the vessel, researchers remove small aliquots at regular intervals that will later be analyzed via HPLC. Furthermore, fully automated on-line systems directly inject the sample into an HPLC system and offer in situ reaction monitoring capabilities.
Dissolution testing systems are used in a variety of laboratory settings including R&D, quality control, and production steps of pharmaceutical development. The systems must be durable and provide quality information to meet regulatory requirements. In addition, because dissolution testing is so important to drug development, it is an essential tool for pharmaceutical companies.
The pharmaceutical industry accounts for an overwhelming majority of the market; however, this industry is not the only consumer of dissolution testing instruments. Other businesses, such as contract research organizations (CROs), in addition to biotechnology, agriculture and food, and academic laboratories, account for the remaining share of the market.
The foregoing data were extracted and adapted from SDi's recently published Global Assessment Report, 12th Edition. For more information, contact Glenn Cudiamat, VP of Research Services, Strategic Directions International, Inc., 6242 Westchester Parkway, Suite 100, Los Angeles, CA 90045, (310) 641-4982, fax: (310) 641-8851, email: cudiamat@strategic-directions.com
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Top Execs from Agilent, Waters, and Bruker Take the Stage at J.P. Morgan Healthcare Conference
January 16th 2025The 43rd Annual Healthcare J.P. Morgan Healthcare Conference kicked off in San Francisco earlier this week. Here’s what top executives from Agilent, Bruker, and Waters, discussed during the event.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

