Market Profile: Chromatography Data Systems
A chromatography data system (CDS) is a software program that automates instrument control, data acquisition, data processing, data filtering, data storage, and database management for ion chromatography (IC), high performance liquid chromatography (HPLC), gas chromatography (GC), supercritical fluid chromatography (SFC), and capillary electrophoresis (CE) instrumentation.
A chromatography data system (CDS) is a software program that automates instrument control, data acquisition, data processing, data filtering, data storage, and database management for ion chromatography (IC), high performance liquid chromatography (HPLC), gas chromatography (GC), supercritical fluid chromatography (SFC), and capillary electrophoresis (CE) instrumentation. A CDS allows multi-instrument control and multi-user access for use in secure and network environments. End-users are able to control menu management, forms display, validation, data entry, text editing, data preprocessing, and generation of reports.
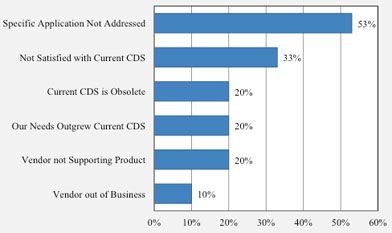
Reasons for replacing current CDS
Two types of CDS architectures are available: workstation (standalone) and client–server (multiuser). A workstation architecture allows CDS software to be run on only one PC and is only able to control up to five instruments. Aclient–server architecture offers multiuser capability, increased data storage, and a database. This architecture usually involves a dedicated server networked with several PCs or terminals that serve as clients. In addition, one or more servers or PCs can be easily added to the network.
In a recent SDi survey, a total of 105 CDS users were asked to express their views and opinions on a wide variety of aspects of chromatography data systems. Respondents were questioned about the technologies they use, vendor knowledge and satisfaction, and future trends regarding CDS. Of the participants who indicated plans to replace their current chromatography data system, more than half indicated that they would do so because their specific application is not being addressed by their current system. About one-third also indicated that their lack of satisfaction with their current CDS is also a reason for replacement. Other reasons mentioned include obsolete CDS, needs outgrew current system, the lack of product support from current vendor, and current CDS vendor out of business.
The foregoing data were extracted and adapted from SDi’s recently published Market Analysis and Perspective (MAP) report titled High Performance Liquid Chromatography: Competing Techniques Setting the Bar Higher. For more information, contact Glenn Cudiamat, VP of Research Services, Strategic Directions International, Inc., 6242 Westchester Parkway, Suite 100, Los Angeles, CA 90045, (310) 641-4982, fax: (310) 641-8851, e-mail: cudiamat@strategic-directions.com
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.
Top Execs from Agilent, Waters, and Bruker Take the Stage at J.P. Morgan Healthcare Conference
January 16th 2025The 43rd Annual Healthcare J.P. Morgan Healthcare Conference kicked off in San Francisco earlier this week. Here’s what top executives from Agilent, Bruker, and Waters, discussed during the event.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

