Low-Level Determination of Mutagenic Nitrosamine Impurities in Drug Substances by LC–MS/MS
Since the detection of N-nitrosodimethylamine (NDMA) in a batch of valsartan in 2018, at levels exceeding ICH acceptable intake limits for mutagenic impurities, the analysis of nitrosamines has become an intense focus point for the pharmaceutical industry. The identification and low-level determination of nitrosamines in potentially affected materials is challenging and requires the application of highly sensitive analytical techniques. This article reviews the chronological development of the story and the regulatory landscape that has evolved. It will then discuss the development of analytical methods for the determination of a series of nitrosamines referenced by regulatory authorities, demonstrating separation of these compounds from the active pharmaceutical ingredient (API) and looking at how mass spectrometry (MS) can be applied to ensure that the required detection limits can be reached.

In the summer of 2018, the pharmaceutical landscape for the manufacture of small molecules changed with the discovery of a small mutagenic compound in a batch of pharmaceuticals manufactured in China. Zhejiang Huahai Pharmaceuticals produce valsartan, which is a prescription-only drug used to treat high blood pressure and heart failure. It is a selective angiotensin II receptor blocker (ARB) that dilates blood vessels and so consequently reduces blood pressure. It is often given to patients directly after a heart attack. In performing the routine analysis, quality control (QC) chemists identified the presence of N-nitrosodimethylamine (NDMA) and went on to report an average level of 66.5 parts per million in affected batches (1), which is high enough to have a detrimental impact on patient safety. On 5 July 2018, the European Medical Agency gave notice on their website that it was reviewing medicines containing valsartan from Zhejiang Huahai following detection of an impurity. It was also noted that national authorities across the EU had started to recall medicines containing valsartan from Zhejiang Huahai. Since 2018, NDMA and other forms of nitrosamines have been observed in a range of different pharmaceuticals. This article will look at the chronological development of the story and how nitrosamines have caused the pharmaceutical industry to re-evaluate their manufacturing procedures. It will look at the synthetic pathways that can cause the generation of a range of nitrosamines and the regulatory landscape that has evolved as a consequence of the initial findings. The article will then discuss the development of a series of applications that will allow for the determination of a series of nitrosamines referenced by regulatory authorities, initially showing how to perform a separation of these compounds from the active pharmaceutical ingredient (API) and then looking at how mass spectrometry (MS) can be applied to the analysis to ensure that the required detection limits can be reached.
The role of the quality control (QC) department is to check the quality of a final product and, in the case of the pharmaceutical industry, there are two key aspects that the QC department will monitor:
- The amount of active ingredient to ensure that the drug is efficacious,
- The amount and type of impurities to ensure that the drug is safe to use.
These analyses can be subdivided into a range of activities, with separation science being a fundamental component of the process.
Impurities that are separated from the major component above a certain level as defined by the regulatory bodies must be identified and then quantified in subsequent analyses of the final product. Figure 1 outlines the decision tree that is used by pharmaceutical organizations
to determine if further investigation is required to determine the impact (2).
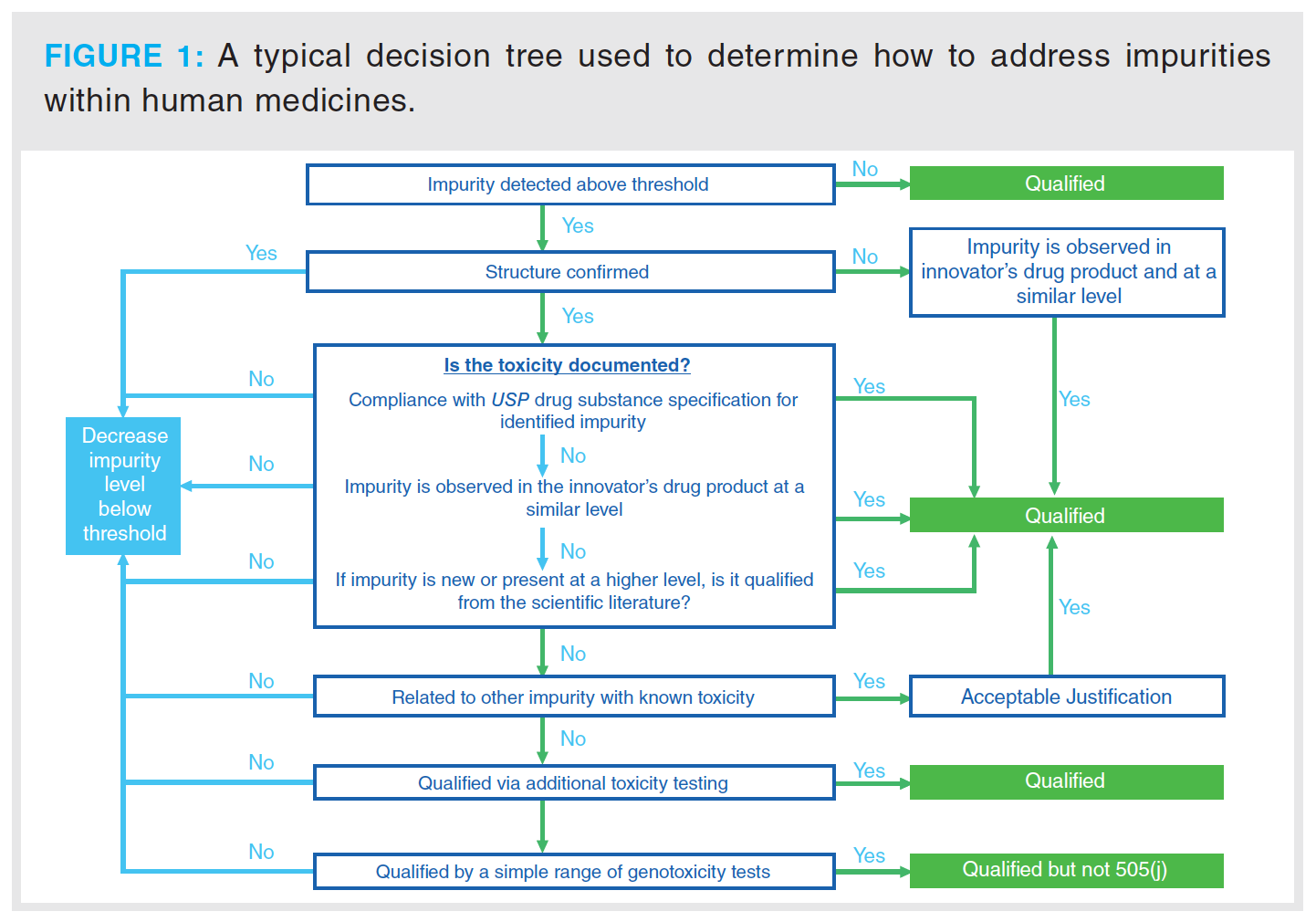
This decision process is associated with a new drug application (NDA), although for the testing of a generic drug, where the process is defined as an abbreviated new drug application (ANDA), the process would be different in accordance with Section 505(j) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (2), in which case the applicant is looking to demonstrate the drug is equivalent to a reference listed drug (RLD).
The regulations stipulate the detection levels that are required for various types of impurities that are identified through the drug development process. For example, for drug substances with a maximum daily dose ≤ 2 g/day, any impurity detected that is nominally above 0.1% requires structural identification. Any impurities exceeding the 0.15% qualification threshold require further assessment to determine biological safety (3). The next course of action will be very dependent on the outcome of this and will determine if the drug product is suitable for release.
In the summer of 2018, it was separation science (with mass spectrometry [MS] identification and quantification) that isolated a small mutagenic compound, a nitrosamine, in a batch of valsartan. Mutagens are chemicals that damage the genetic information within a cell, resulting in mutations that may lead to cancer. The damage to the cell is caused by interactions with the DNA sequence and the DNA structure. The alteration to the DNA may also result in permanent heritable changes to the somatic cells of the organism or germ cells that can then be passed on to future generations. It is clearly important that mutagenic compounds can be detected and their production avoided wherever possible. The bacterial reverse mutation assay, also referred to as the Ames assay (4), is often used to test for gene mutation and it has been used to identify a range of chemical entities that have been reported to be mutagenic (5), including nitrosamines. The World Health Organization has used the principles outlined in the ICH M7(R1) (6) guideline to determine an acceptable daily intake. This information is used to determine the allowable daily intake of mutagenic impurities for a specific medication, as will be detailed later in this article.
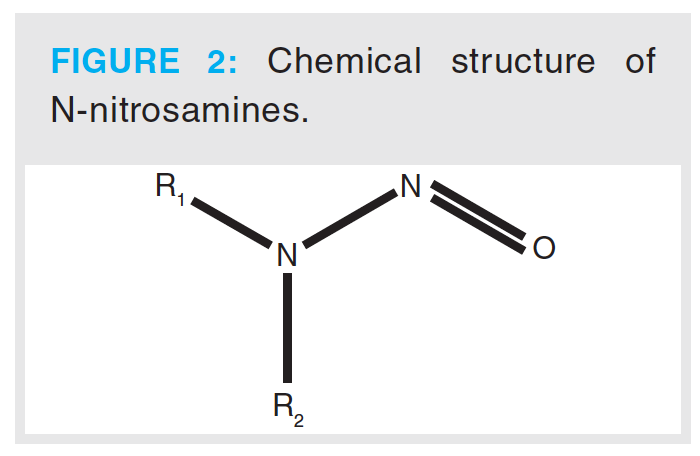
Nitrosamines are a class of compounds that have a chemical structure containing a nitroso group bonded to an amine, as shown in Figure 2. The toxic properties of nitrosamines were first reported by Barnes and Magee, who reported that N-nitrosodimethylamine (NDMA) produced liver tumours in rats. Subsequent studies, which involved the testing of over 300 nitrosamines, showed that nearly 90% of these compounds were carcinogenic to a wide variety of animals (7). They have been identified in a variety of sources, including environmental sources, drinking water, and processed food products. The potential for formation in pharmaceutical drug products, such as aminophenazone, in the presence of nitrosating agents was established in the 1970s. In the literature, several other active pharmaceutical ingredients (APIs) have also been reported to contain NDMA, including aminopyrimidine, amitriptyline, chloramphenicol, oxytetracycline, promazine, propoxyphene, chlorpromazine, diphenhydramine, doxylamine, trimipramine, tetracycline, erythromycin, imipramine, and methapyrilene (8,9). In 2018, the detection of high levels of NDMA in batches of valsartan drug product prompted renewed focus on this class of impurity.
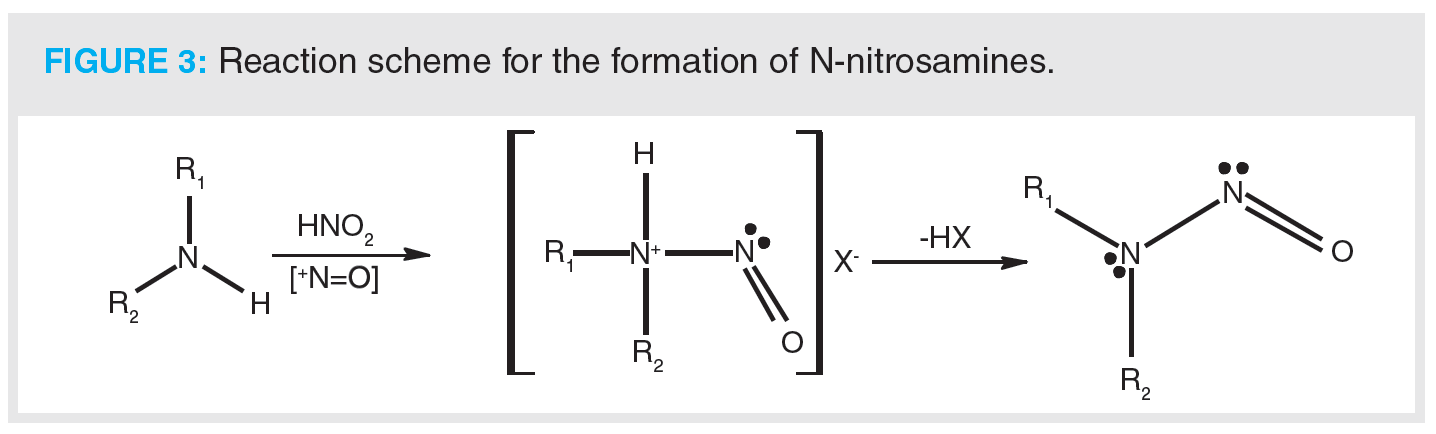
Nitrosamines can be formed by the reaction of a secondary amine with nitrite (Figure 3). Therefore, it is suspected that NDMA in valsartan may come from the ZnCl2-catalyzed disproportionation of dimethylformamide (DMF) to dimethylamine and carbon monoxide (CO). Dimethylamine then goes on to react with sodium nitrite and the resulting product is NDMA. It has also been proposed that carryover of nitrites or amines from subsequent steps may also result in the formation. Notably, contamination from external sources has been highlighted as a potential source of nitrosamines (1), such as the use of recycled solvents, for example DMF—which is quenched with sodium nitrite to destroy residual azide as part of the recovery process—is thought to be a potentially significant source of NDMA. Recycling of materials, catalysts, and solvents is deemed to be a major factor in the formation of nitrosamines, along with impurities that could react further downstream if equipment is not adequately cleaned. Similar reaction schemes have been proposed for the formation of other nitrosamines that have been identified during impurity profiling. An additional theoretical source of nitrosamines that should be considered is the formation of higher molecular weight API-like nitrosamines within a drug product. In the case of valsartan, two valsartan-specific nitrosamines were confirmed by a single API manufacturer to the European Medicines Agency (EMA), albeit at acceptably low levels (8).
Regulatory authorities first became aware of the presence of nitrosamines, specifically NDMA, in pharmaceutical product in July 2018. It was first detected in an angiotensin II receptor blocker, valsartan, and since then other nitrosamines have been detected in other sartan drug products that contain a tetrazole ring, along with other API/medicinal products, including ranitidine and pioglitazone. The FDA and EMA have highlighted several nitrosamines that could be generated during the production process as potentially existing within drug products. These are highlighted in Table 1, with the required acceptable intake limits as specified by the regulatory authorities (8,10,11).
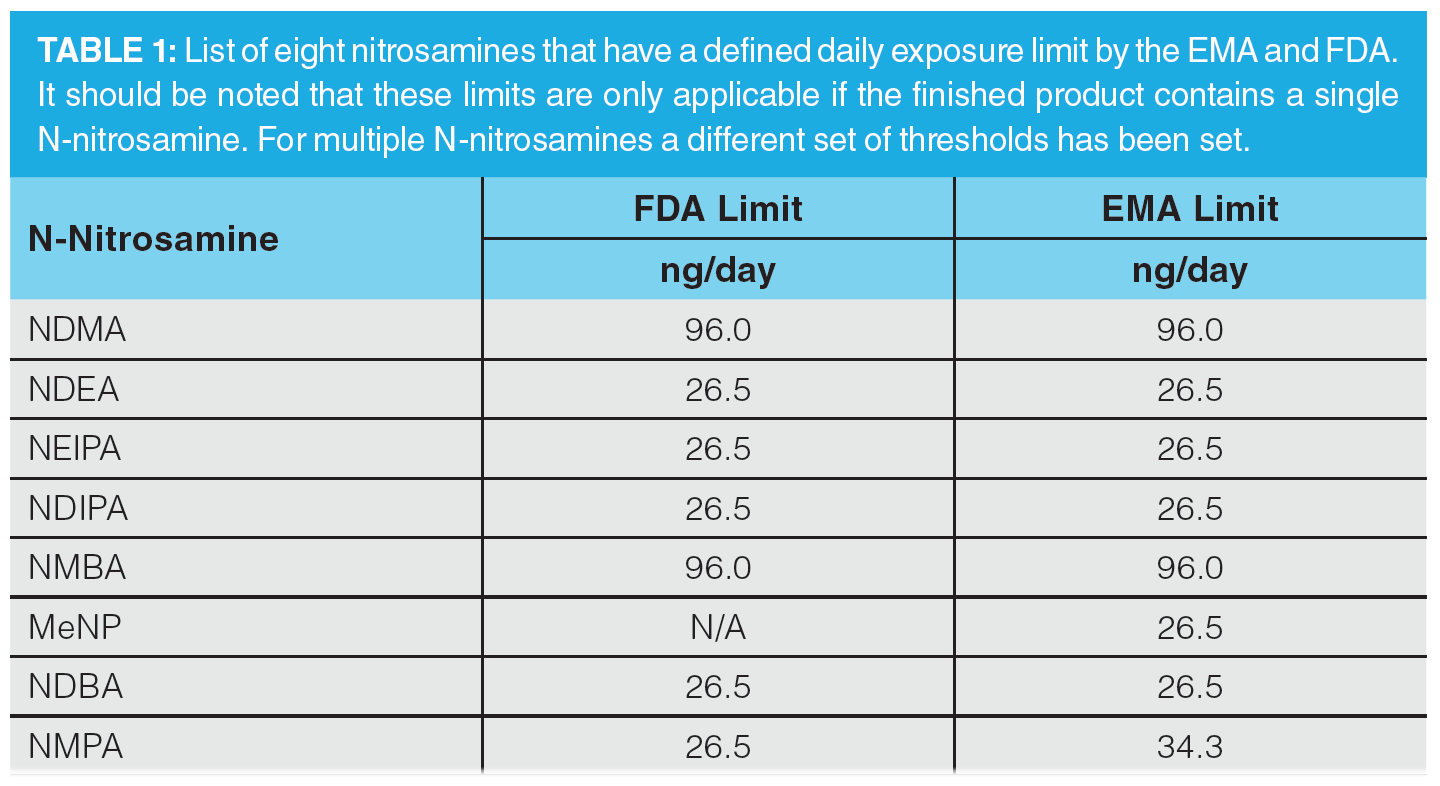
The nitrosamines specifically highlighted as of being of concern are: N-nitrosodimethylamine (NDMA), N-nitrosodiethylamine (NDEA), N-nitrosoethylisopropylamine (NEIPA), N-nitroso-diisopropylamine (NDIPA), N -nitroso-N-methyl-4-aminobutyric acid (NMBA), 1-nitroso-4-methyl piperazine (MeNP), N-Nitrosodibutylamine (NDBA), and N-nitrosomethylphenylamine (NMPA). It should be noted though that both regulatory authorities do stipulate that any carcinogenic impurity should be assessed in accordance with the ICH M7(R1) guidelines, and indeed the EMA documentation refers to 16 different nitrosamines that are routinely monitored under the United States Environmental Protection Agency (EPA) guidelines (8,12). Separation and detection of these impurities within solvents, intermediates, drug substance, and drug product has therefore become of high importance within the pharmaceutical industry.
The regulatory landscape has evolved very quickly since the first observation of NDMA in valsartan, with the FDA releasing documentation related to controlling nitrosamine impurities in human drugs in September 2020, which was recently updated in February 2021 (10). There has also been legislation released by the EMA that defines the allowable daily intakes. This information can be used to determine the allowable concentrations in a given drug product (8). Current acceptable intake limits required by the pharmaceutical industry are given in Table 2. The acceptable daily intake of nitrosamines is related to the maximum daily dose (MDD) of the drug substance, therefore different concentrations of nitrosamines are acceptable for different drugs.
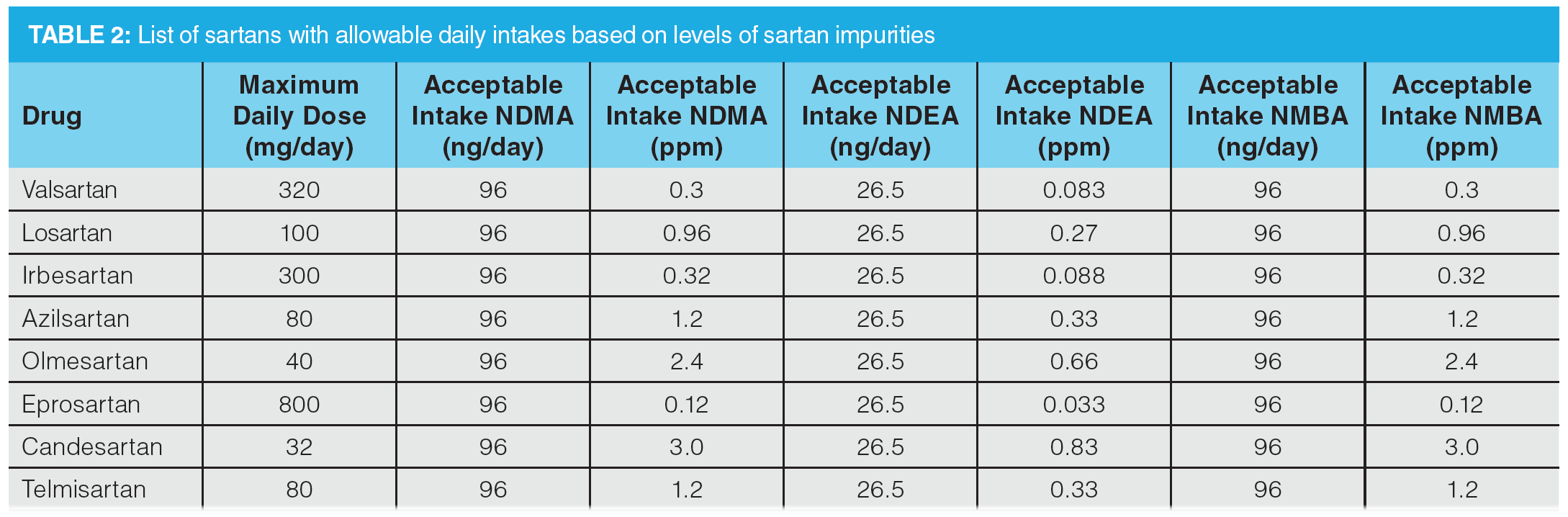
These limits are applicable only if a drug product contains a single nitrosamine. If more than one of the nitrosamine impurities identified in Table 1 is detected and the total quantity of nitrosamine impurities exceeds 26.5 ng/day (the acceptable intake [AI] for the most potent nitrosamines) based on the MDD (10), the manufacturer should contact the agency for evaluation. For drug products with an MDD of less than 880 mg/day, a recommended limit for total nitrosamines of 0.03 ppm is no more than 26.5 ng/day and is considered acceptable. For drug products with an MDD above 880 mg/day, the limit for total nitrosamines should be adjusted so as not to exceed the recommended limit of 26.5 ng/day. The wording here is taken directly from the February 2021 guidance to industry document on “Control of Nitrosamine Impurities in Human Drugs” (10). In Europe, the total risk level for all N-nitrosamines detected must not exceed 1 in 100,000 lifetime risk. An alternative approach where the sum of all detected N-nitrosamines is below the limit of the most potent nitrosamine may also be used (8,11). This clearly demonstrates the complexity of developing an analytical method for the analysis of nitrosamines in an API, since there is a degree of interpretation required to define the assay dynamic range and this varies depending on the nature of the nitrosamine impurities being analyzed.
It is very evident from the above discussion that since the initial identification of NDMA in valsartan that there has been a lot of activity both in terms of identification of nitrosamine impurities and in terms of the required legislation to address the potential toxicity implications. Figure 4 gives an overview of the main activities associated with this unfolding story from the initial discovery in June 2018, through the triggering of article 31, which is when the EMA initiated non-pharmacovigilance referral procedures, to the more recent guidance updates by the FDA on the analysis of nitrosamines and the more recent observations of nitrosamines in other APIs.
Materials and Methods
Liquid chromatography (LC)–UV experimental work was performed on an Agilent 1290 binary UHPLC system, with data acquisition and analysis performed using Agilent OpenLab CDS and ChemStation software (Agilent Technologies). The LC–MS application was run on a Sciex QTRAP 6500+ LC–MS/MS system equipped with an ExionLC AD UHPLC system (Sciex). The MS was operated in positive ionization mode using the atmospheric pressure chemical ionization (APCI) probe on the IonDrive Turbo V source. Data acquisition was managed by Sciex Analyst software, whilst data was processed using Sciex OS. The LC separations were performed on Avantor ACE UltraCore C18 superficially porous columns in 100 × 3.0 mm and 100 × 2.1 mm dimensions and a 3.5‑µm particle size (VWR). Solvents and reagents were purchased from VWR. NDMA, NDEA, NPIDA, NDPA, NDBA, NMPA, NDMA-d6, and valsartan standards were purchased from VWR, NMBA and NEIPA from Enamine Ltd, and NMBA-d3, NDEA-d10, and NDBA-d18 from LGC Ltd.
For the LC–MS application, a standard solution containing 200 ng/mL of NDMA, NMBA, NEIPA, NDIPA, NDPA, NMPA, and NDBA was prepared, along with a 132 ng/mL solution of NDEA. The internal standard solution contained 10 µg/mL NDMA-d6 and NMBA-d3 and 1 µg/mL NDEA-d10 and NDBA-d18. L1–L7 calibration solutions were prepared as outlined in the proposed USP method (13). The spiked valsartan sample was prepared by spiking 80 mg of drug substance with 6 µL and 9 µL of the respective standard solutions and 12 µL of internal standard solution, followed by 1173 µL 1% formic acid (aq.). The sample was vortexed at 2500 rpm for 20 min and then centrifuged at 6000 rpm for 20 min. It was then filtered using a Whatman Mini‑UniPrep 0.45 µm PVDF syringeless filter.
Chromatographic Analysis of Nitrosamines
The low-level detection of nitrosamines including NDMA is highly pertinent due to potential trace-level formation in the manufacture and processing of various consumer products, including drinking water, food and beverages, tobacco‑related products, rubber products, pharmaceuticals, and cosmetics (8,14,15,16). The potential for the formation of nitrosamines in such products, for example, the reaction of nitrite with nitrosatable amines in meat and fish at high temperatures, has caused major concern. The chromatographic separation and quantitation of nitrosamines is therefore well established in a variety of matrices using both gas chromatography (GC) or LC. GC is ideally suited to the analysis of more volatile nitrosamines and has been utilized extensively. Several detection options have been explored for GC analysis of nitrosamines. Nitrogen chemiluminescence detection (CLD), also termed thermal energy analyzer (TEA), has been used to provide a highly sensitive detection method for N-nitrosamines (9,17); however, potential for cross reactivity exists from organic and inorganic nitrites, nitrates, and N-nitramines (8). The use of MS detection has become increasingly established, providing highly sensitive, analyte-specific detection capabilities.
Liquid chromatography is also commonly used and can overcome some reported issues found with GC analysis, for example, allowing determination of thermally unstable nitrosamines (14) and involatile nitrosamines such as NMBA, and avoiding potential thermal degradation of ranitidine drug substances under GC conditions to yield NDMA (18). LC also enables the simultaneous determination of nitrosamines alongside drug product in a single run. The separation of nitrosamines can be achieved by reversed-phase LC, although given the hydrophilic, polar nature of some nitrosamines, retention of some of the compounds can be challenging. Various stationary phases have been utilized to obtain satisfactory retention, with C18 or pentafluorophenyl (PFP) phases often specified. The proposed USP general chapter on nitrosamine impurities, currently under review (April 2021), provides monograph methods for the use of both these LC phases (13). The FDA additionally specifies the use of a C18 phase with an integrated phenyl ring for the determination of NDMA in ranitidine (19,20).
To demonstrate the applicability of LC for the separation of nitrosamine impurities in pharmaceutical preparations, a set of eight nitrosamines were spiked into a valsartan secondary standard and separated on a 100 × 3.0 mm solid-core C18 column (Figure 5). The set of nitrosamine impurities selected for this example include the majority of the nitrosamines identified as being of concern by the USP, EMA, and FDA (8,10,13). The sample analytes possess log P values ranging from -0.17 to 2.60, therefore gradient elution was required to provide suitable retention of all analytes. A simple gradient ranging from 4 to 95% B (2.8% to 66.5% [v/v] acetonitrile) was found to achieve baseline separation of all sample components. An initial 1 min isocratic hold was employed to aid retention of NDMA. It is worth noting that the peaks for asymmetric NMBA and NEIPA are observed as doublet peaks of the syn- and anti-conformers due to hindered rotation around the N-N bond (21). From assessment of the analyte physicochemical properties, mobile phases buffered with potassium phosphate at pH 2.7 and 6.5 were assessed during method development. It was found that an acidic pH was required to improve the retention and peak shape of the valsartan API peak and to also achieve retention of NMBA, with the NMBA peak eluting on the column void time at intermediate pH. In this example, UV detection was employed at a wavelength of 254 nm.
Whilst the example shown in Figure 5 clearly demonstrates the applicability of a C18 phase for the separation of these nitrosamine impurities, the low detection limits specified by regulatory authorities typically precludes the use of UV detection. Nitrosamine analysis therefore requires the use of highly sensitive and specific detection modes, such as MS, although the low molecular weight of nitrosamines and their relatively low hydrophobicity can make developing a sensitive and robust method somewhat challenging. The use of LC–MS/MS has become increasingly common, with nitrosamine detection, quantitation, and confirmation achieved through monitoring multiple analyte specific MRM transitions. Alternatively, LC–high resolution MS (LC–HRMS) has also been employed to provide highly specific detection and quantitation.
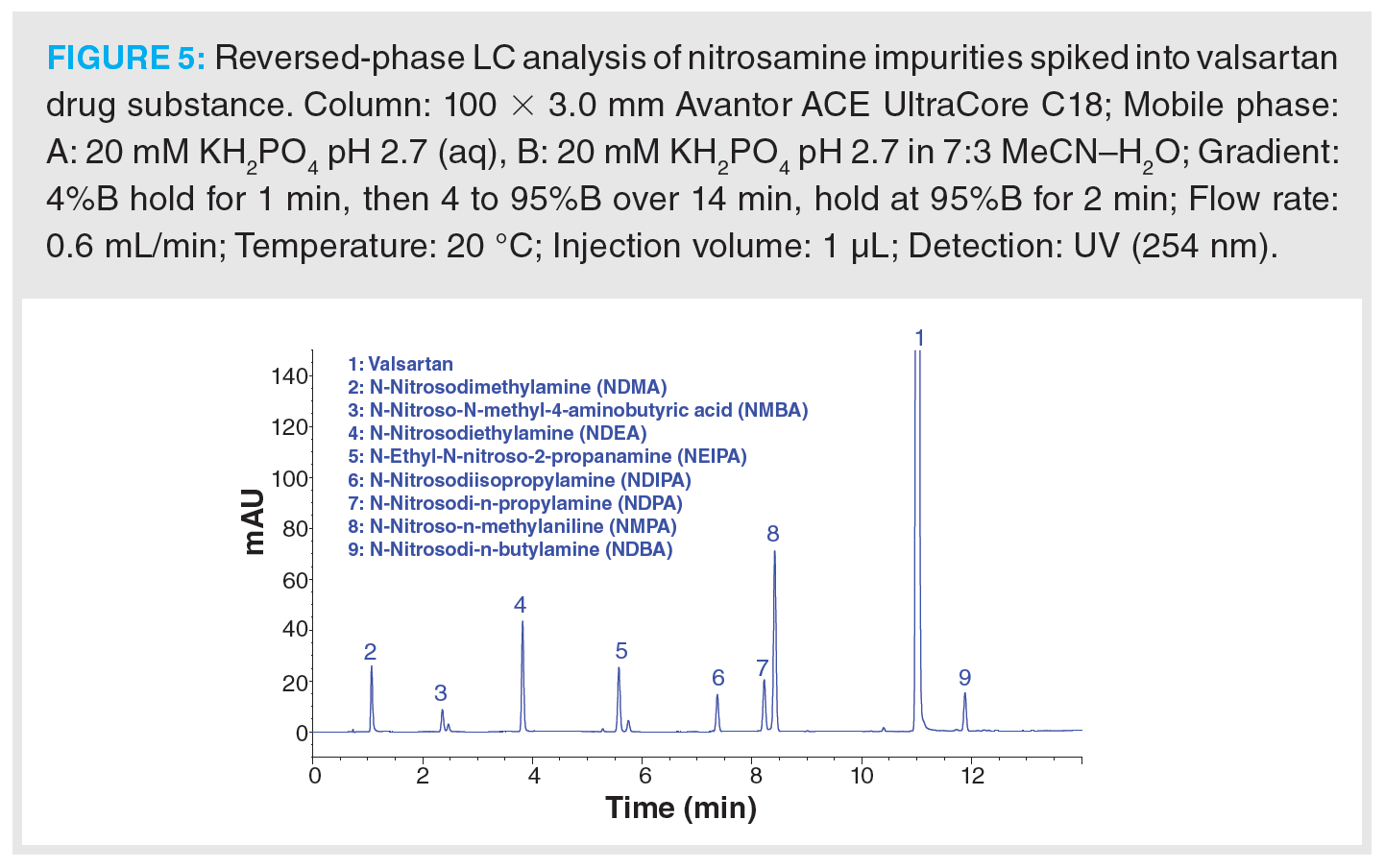
As discussed above, an acidic mobile phase provides good retention and selectivity for nitrosamines, although the nonvolatile phosphate buffer used in Figure 5 should be replaced with an MS-compatible alternative. Formic acid is a convenient additive that is widely used in the mobile phase for nitrosamine analysis by LC–MS. Ngongang et al. reported an increase in MS signal intensity for five out of nine nitrosamines tested over non‑buffered mobile phase, when 0.05 or 0.1% (v/v) formic acid was added to the mobile phase, with 0.1% proving optimal for lower response nitrosamines (14). Higher concentrations of formic acid were found to compromise signal intensity. Electrospray ionization (ESI) has been used for LC–MS analysis of nitrosamines, however, it has been reported that ESI may be impacted by ion suppression due to matrix effects. Therefore, positive mode atmospheric pressure chemical ionization (APCI) is preferential for nitrosamine analysis, providing much improved sensitivity (8,22).
To demonstrate the use of the same stationary phase in Figure 5 for the high sensitivity LC–MS/MS quantitation of nitrosamines in drug substances, an LC–MS/MS method for the determination of six nitrosamines (NDMA, NDEA, NMBA, NEIPA, NDIPA, NDBA) in selected sartans—specified in the proposed USP general chapter on the analysis of nitrosamine impurities—was used as a starting point (13). The monograph method specified the use of a 150 × 3.0 mm column packed with 2.7-µm particles bonded with a C18 phase. A 12.2 min gradient profile was used, which would give an approximate cycle time of 18–24 min (assuming 5–10 column volumes of mobile phase for post‑gradient re-equilibration). Given the high resolution obtained using the LC method in Figure 5, it was decided to develop an alternative gradient method targeting a >50% reduction in overall cycle time. In addition to the six nitrosamines specified in the USP method, NMPA (identified as a nitrosamine of concern by EMA and FDA) and NDPA (isobaric to NDIPA) were also included. The method should provide full chromatographic separation of all analytes, in particular the isobaric pair NDPA and NDIPA. Resolution of the syn- and anti‑conformers for NMBA and NEIPA was not deemed to be necessary, as these would be integrated as a single peak. As per the USP methodology, four deuterated internal standards were included for quantitation: NDMA-d6, NMBA-d3, NDEA-d10, and NDBA-d18 (NEIPA, NDIPA, NDPA, and NMPA are quantitated against NDEA-d10). As shown in Table 2, analytical methods for nitrosamine determination in drug substances require low ppb detection limits to ensure applicability for high daily dose drug substances.
The individual transitions specified in the USP method for quantitation and confirmation were assessed by direct infusion of reference standard solutions of each compound and were either confirmed or replaced by higher performing transitions. The individual transitions were optimized by infusion, as detailed in Table 3, whilst MS source parameters were optimized by flow injection analysis, targeting maximization of the responses for NDMA and NMBA (Table 4).
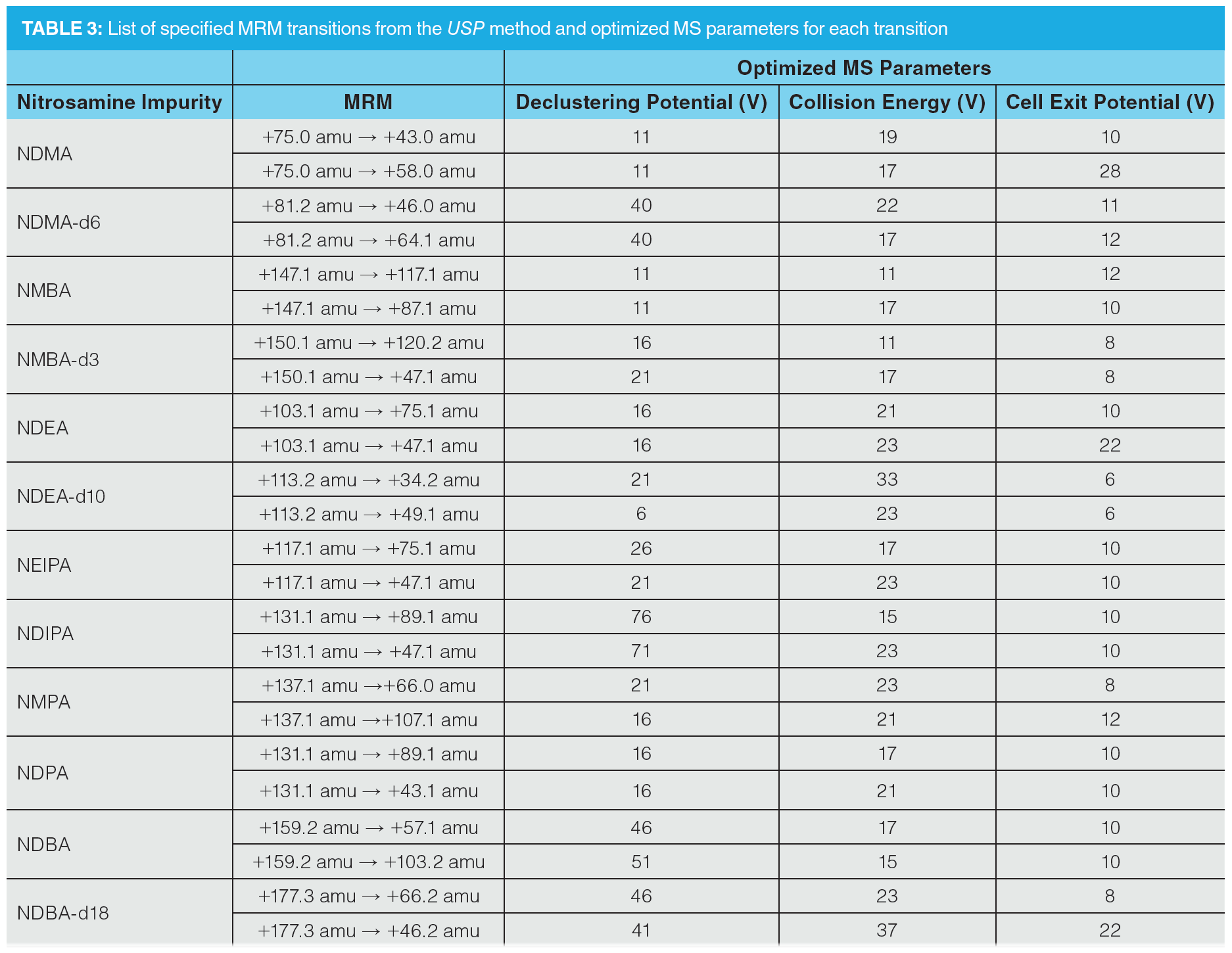
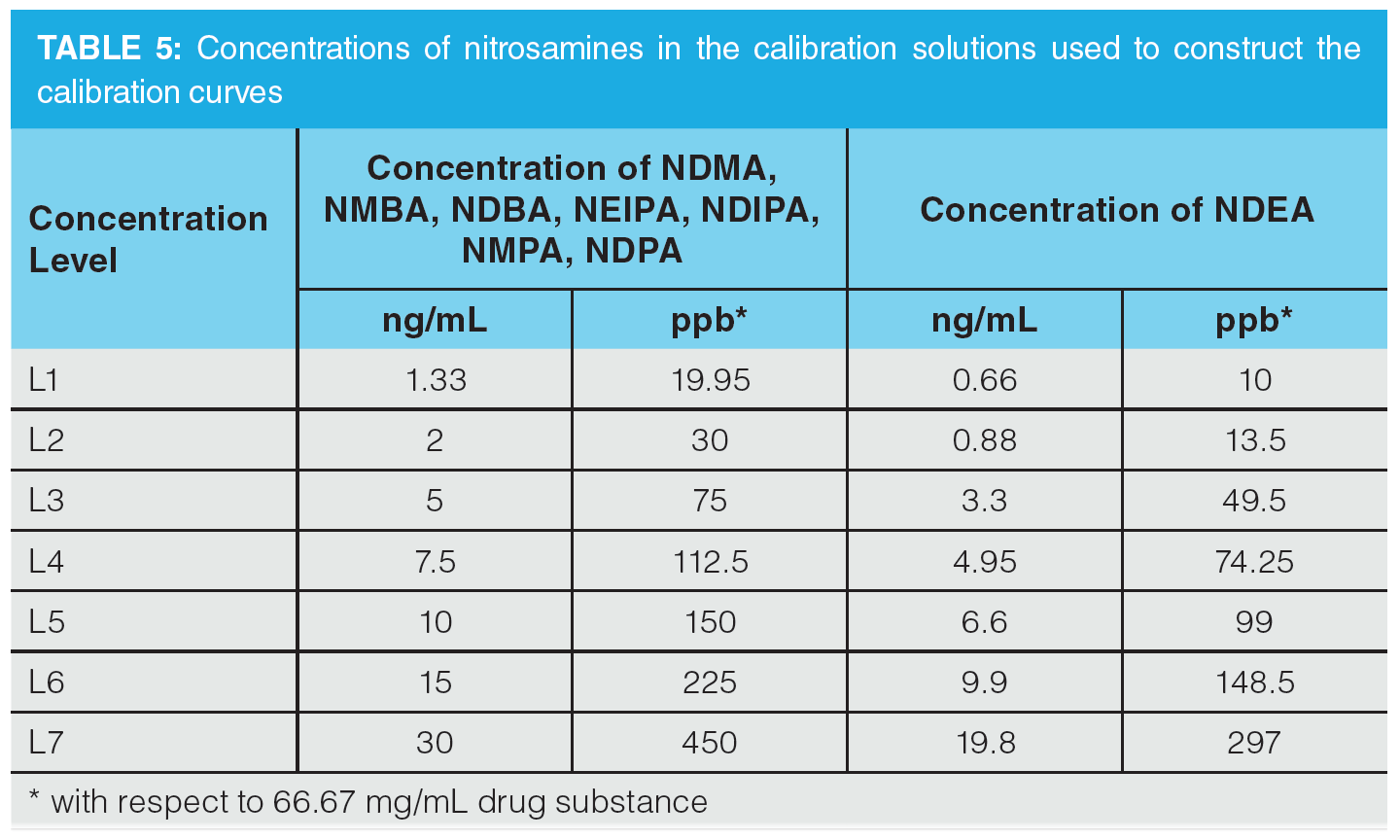
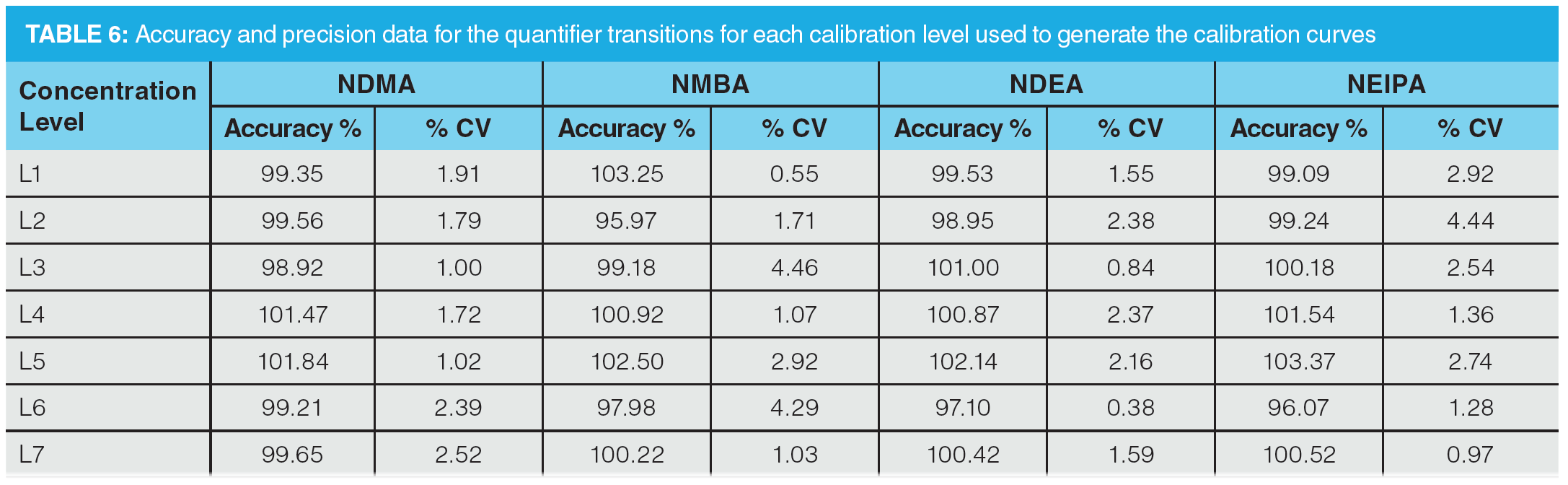
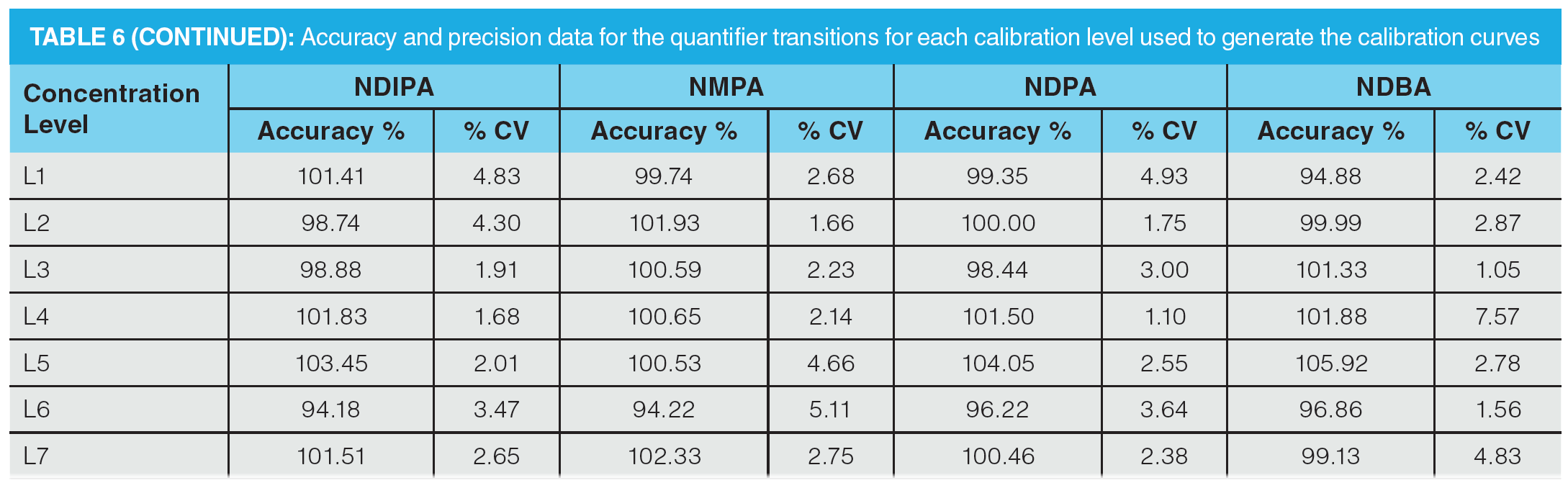
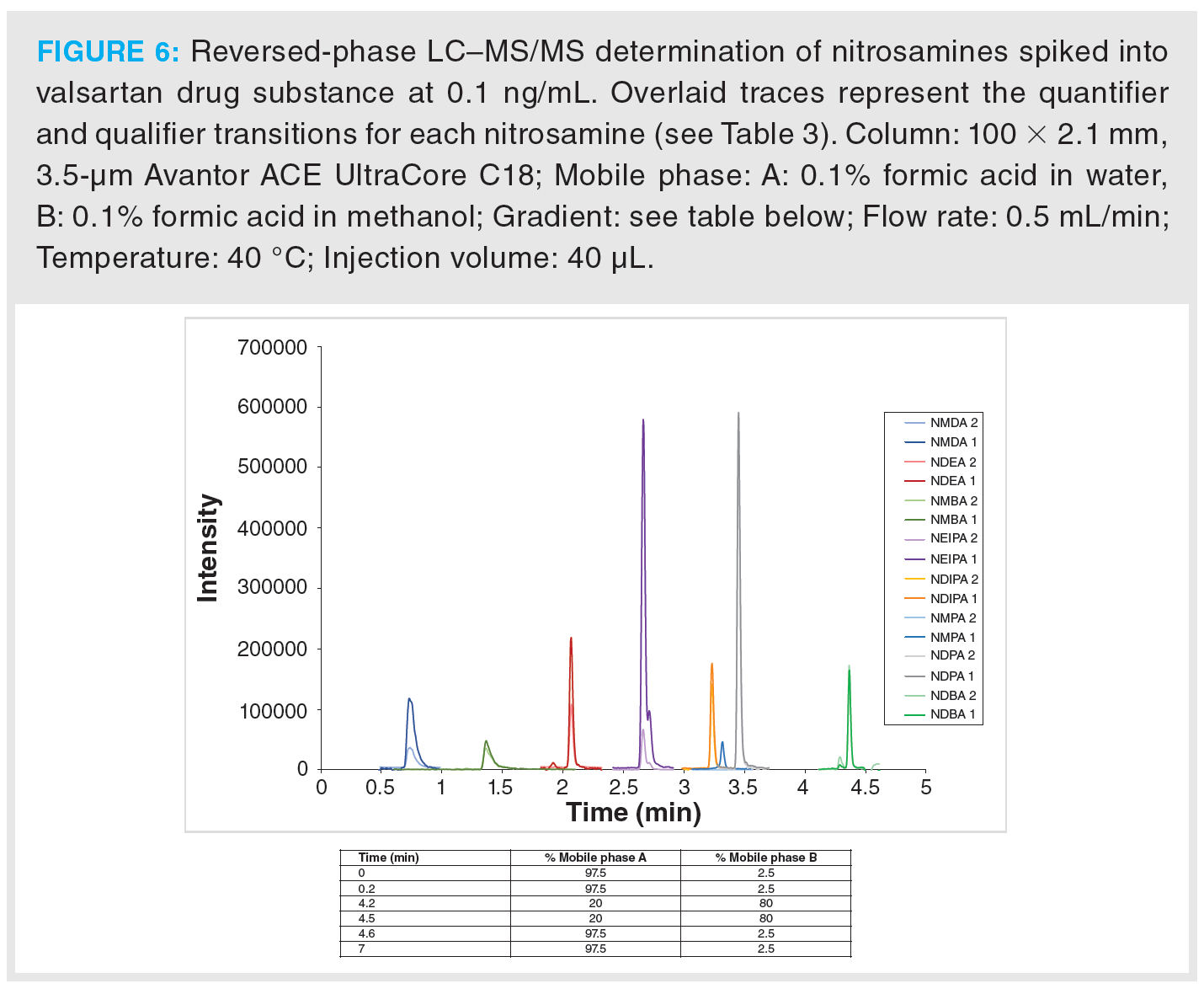
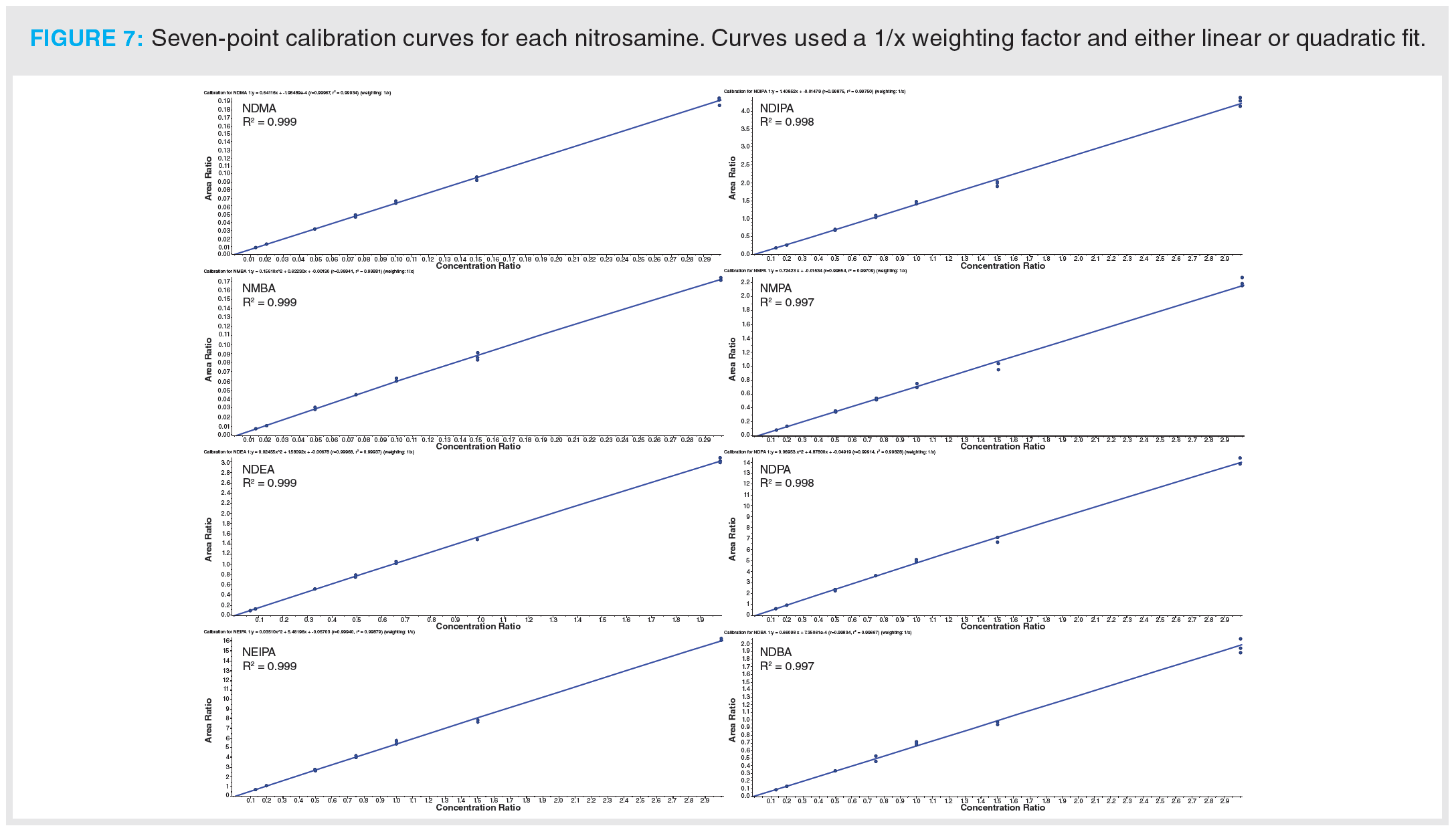
Once optimization was complete, the final LC separation was refined on a 100 × 2.1 mm solid-core C18 column. An initial isocratic hold of 0.2 min was used to maximize retention of NDMA and NMBA, whilst the remaining analytes were eluted on a 4.0 min gradient (full LC conditions are detailed in Figure 6). Seven-point calibration curves were constructed by injection of standard solutions of nitrosamines according to the USP method (Table 5 and Figure 7). Excellent linearity was observed across the calibration range for all nitrosamine standards, with all compounds giving r2 > 0.99, in compliance with method system suitability criteria. The accuracy and precision for the method was very good, as detailed in Table 6, demonstrating reproducibility across the calibrated range.
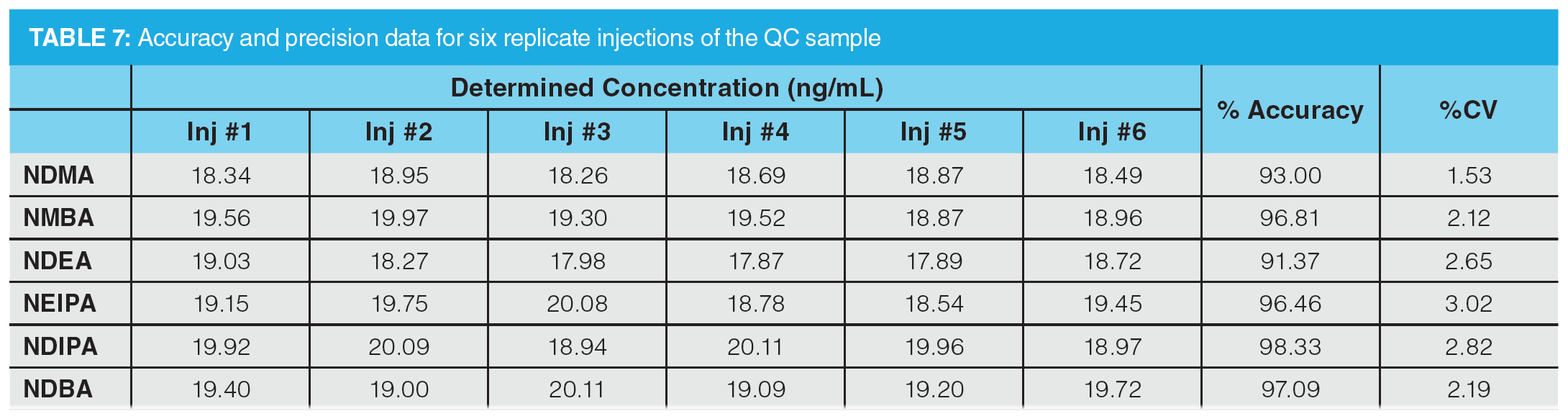
To further assess accuracy and precision, six injections of a QC sample containing the six nitrosamines specified in the USP method were performed (summarized in Table 7). The QC standard was prepared at a concentration of 20 ng/mL, which would correspond to 0.3 ppm with respect to an 80 mg sample of drug substance (AI limit for NDMA and NMBA in valsartan). Determined accuracies were within 15% of the expected range, with %CV values substantially lower than 15%, demonstrating excellent reproducibility.
Finally, the method was applied to the analysis of valsartan drug substance spiked with nitrosamine standard solutions at a level of 1 ng/mL (equating to 15 ppb of drug substance, according to the sample preparation procedure). A simple extraction of nitrosamines with 1% aqueous formic acid was used following reference 11, which successfully extracted the spiked nitrosamines from valsartan drug substance. For the analysis of other drug substances, such as other sartans, ranitidine, or metformin, alternative extraction methods may be required to achieve suitable recovery whilst preventing solubilization of the API (18,23,24).
The analysis of finished drug product (that is, drug substance and excipients) presents further analytical challenges, particularly for drug products with a high MDD. The potential for interference from drug substance or excipients and the low detection limits required means that in some cases sample cleanup and concentration approaches, such as solid-phase extraction (SPE), may need to be employed to mitigate the impact of the matrix (18,25). Additionally, the potential interference from other low‑molecular‑weight trace impurities, such as residual DMF, has also been noted (26).
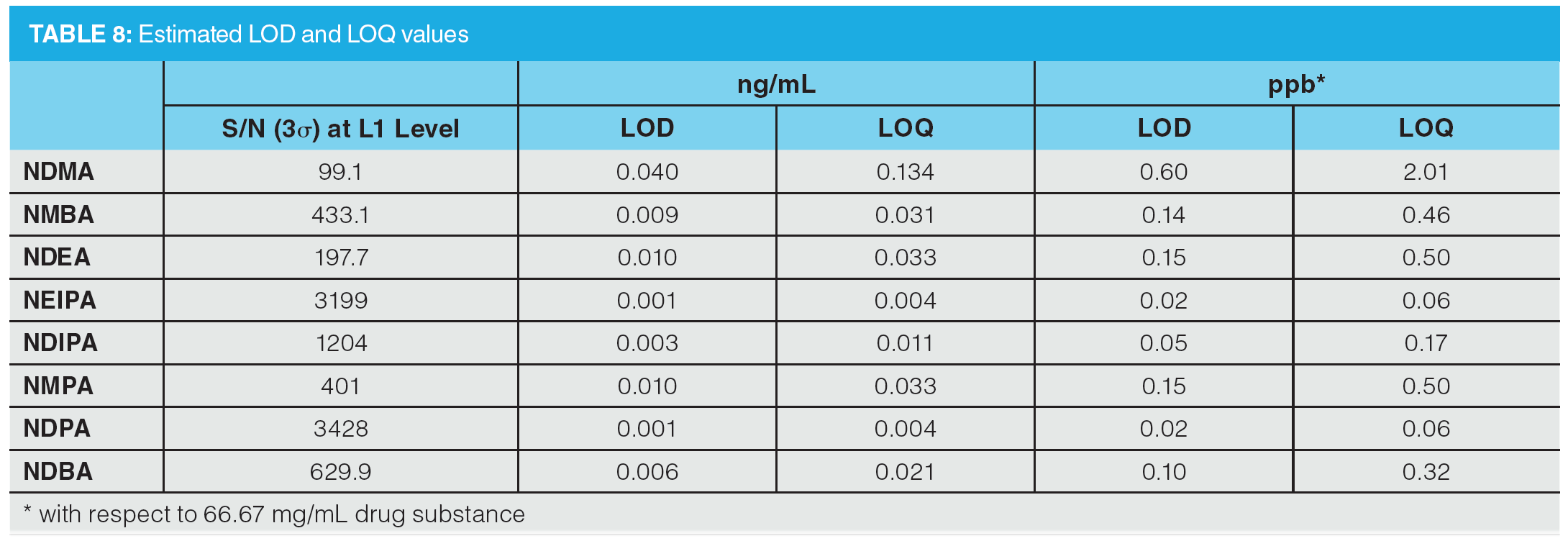
Figure 6 shows an example MRM chromatogram. Chromatographic resolution was obtained for all analytes and the isobaric analytes NDIPA and NDPA were clearly separated. It is worth noting that the syn- and anti-conformers of NEIPA were partially resolved but were integrated as a single peak for quantitation. According to Table 2, the AI limits for NDEA in a high daily dose drug substance correspond to 33 ppb, therefore the method clearly shows excellent performance in the low ppb ranges required by regulatory authorities. LOD and LOQ values were estimated based on the signal-to-noise ratio determined from individual MRM chromatograms obtained for the L1 calibration levels and are summarized in Table 8. These values clearly demonstrate the low ppb sensitivity that can be achieved using the tested method.
Conclusions
Since the initial discovery of NDMA in valsartan in 2018, the detection and quantitation of nitrosamine impurities in drug substances has become a major concern. Risk-based assessment of the nitrosamine contamination risks has been widely implemented to identify and mitigate potential sources of nitrosamine contamination in pharmaceutical products. Where risks are present, analytical determination of nitrosamines is essential to ensure the ongoing safety of drug products. Due to the low acceptable daily intake limits established for nitrosamines, which must be considered against the maximum API daily dose, chromatographic analysis necessarily involves the application of highly sensitive and selective mass spectrometric detection capable of quantitation at the low ppb level.
This article has discussed the potential origins of nitrosamine contamination in drug substance, the regulatory guidance governing the allowable limits of nitrosamines, and discussed the use of LC–MS/MS for low-level nitrosamine quantitation. A proposed USP method for nitrosamines in sartans was selected as a starting point to develop a rapid method for nitrosamine analysis. The final LC–MS/MS method reduced the overall cycle time by 50%. The excellent separation and peak shape provided by the LC column and the separation conditions employed allow this LC–MS/MS approach to readily provide the sensitivity to quantify nitrosamines at the low levels required by regulatory authorities. The final method was demonstrated to show excellent accuracy and precision across the calibration concentration range assessed.
References
- European Medicines Agency, Lessons learnt from presence of N-nitrosamine impurities in sartan medicines (June 2020, online). Available: https://www.ema.europa.eu/en/documents/report/lessons-learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf (Accessed June 2021).
- US Food and Drug Administration, Guidance for Industry ANDAs: Impurities in Drug Substances (FDA, Rockville, Maryland, USA, 1999, online). Available: https://www.fda.gov/files/drugs/published/ANDA%27s--Impurities-in-Drug-Substances.pdf
- European Medicines Agency, ICH Topic Q 3 A (R2), Impurities in new Drug Substances (October 2006, online). Available: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-3-r2-impurities-new-drug-substances-step-5_en.pdf (Accessed June 2021).
- B.N. Ames, W.E. Durston, E. Yamasaki, et al., P. Natl. Acad. Sci. USA 70(8), 2281–2285 (1973).
- J. McCann, E. Choi, E. Yamasaki, et al., P. Natl. Acad. Sci. USA 72(12), 5135–5139 (1975).
- FDA/ICH, M7(R1) Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk (online) Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/m7r1-assessment-and-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit-potential.
- J. Barnes and P. Magee, Brit. J. Ind. Med. 11(3), 167–174 (1954).
- European Medicines Agency, Nitrosamine impurities in human medicinal products, Procedure number: EMEA/H/A-5(3)/1490 (25 June 2020, online). Available: https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf (Accessed March 2021).
- M.K. Parr and J.F. Joseph, J. Pharm. Biomed. Anal. 164, 536–549 (2019).
- US Food and Drug Administration, Control of nitrosamine impurities in human drugs (FDA, Rockville, Maryland, USA, February 2021, online) Available: https://www.fda.gov/media/141720/download
- European Medicines Agency, Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 referral on nitrosamine impurities in human medicinal products (23 April 2021, online). Available: https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-questions-answers-marketing-authorisation-holders/applicants-chmp-opinion-article-53-regulation-ec-no-726/2004-referral-nitrosamine-impurities-human-medicinal-products_en.pdf (Accessed June 2021).
- United States Environmental Protection Agency https://comptox.epa.gov/dashboard
- United States Pharmacopeia Proposed General Chapter <1469> “Nitrosamine Impurities” (United States Pharmacopeial Convention, Rockville, Maryland, USA, September 2020 ,online). (Accessed 2021 February).
- A.D. Ngongang, S.V. Duy, and S. Sauve, Anal. Methods 7, 5748–5759 (2015).
- S.-J. Park, M.-J. Jeong, S.-R. Park, et al., Food Sci. Biotechnol. 27, 1519–1524 (2018).
- C.-C. Fan and T.-F. Lin, Chemosphere 200, 48–56 (2018).
- A. James, The Column 15(9), 20–24 (2019).
- H.-H. Lim, Y.-S. Oh and H.-S. Shin, J. Pharm. Biomed. Anal. 189, 113460 (2020).
- US Food and Drug Administration, Liquid Chromatography-High Resolution Mass Spectrometry (LC–HRMS) Method for the Determination of NDMA in Ranitidine Drug Substance and Drug Product (FDA, Rockville, Maryland, USA, September 2019, online). Available: https://www.fda.gov/media/130801/download (Accessed March 2021).
- US Food and Drug Administration, Liquid Chromatography-Tandem Mass Spectrometry (LC–MS/MS) Method for the Determination of NDMA in Ranitidine Drug Substance and Solid Dosage Drug Product (FDA, Rockville, Maryland, USA, October 2019, online). Available: https://www.fda.gov/media/131868/download (Accessed March 2021).
- T.W. Iwaoka, T. Hansen, S.T. Hsieh, et al., J. Chrom. A 103, 349–354 (1975).
- J.-H. Lee, S.-U. Lee, and J.E. Oh, Int. J. Environ. Anal. Chem. 93(12), 1261–1273 (2013).
- O. Scherf-Clavel, M. Kinzig, A. Besa, et al., J. Pharm. Biomed. Anal. 171, 278–284 (2019).
- F. Sörgel, M. Kinzig, M. Abdel-Tawab, et al., J. Pharm. Biomed. Anal. 172, 395–405 (2019).
- K.M. Shaik, B. Sarmah, G.S. Wadekar, et al., Crit. Rev. Anal. Chem. 1–19 (2020).
- J. Yang, T. Andres Marzan, W. Ye, et al., AAPS J. 22, 89 (2020).
Matt James is a senior research scientist at Avantor.
Tony Edge is an R&D manager at Avantor, heading a team of specialist scientists in developing next-generation stationary phases for HPLC.

Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










