Leveraging the Power of a Complementary Separation Technology to Tackle Tough Challenges in Pharmaceutical Analysis
Pharmaceutical development relies on a diverse array of tools and techniques to characterize drug products and their biological targets. Among these techniques, high-resolution ion mobility‒mass spectrometry (HRIM-MS) is emerging as a powerful tool for the separation and analysis of compounds that challenge traditional methods, providing valuable insights into complex samples that are otherwise difficult to analyze. A key advantage of HRIM-MS lies in its simplicity and speed in contrast to other recent structural tools and techniques, such as alternate tandem mass spectrometry (MS/MS) fragmentation methods or chemical derivatization. The high peak capacity achieved using HRIM-MS enables a deeper analysis of samples that is often possible in the same or less time compared to traditional methods. HRIM-MS has demonstrated value in examining a wide range of molecules relevant to the pharmaceutical and biopharmaceutical industries. In the analysis of lipid nanoparticles, an increasingly popular delivery vehicle for new drugs, HRIM-MS has been shown to reveal hidden impurities. HRIM-MS has shown unique capabilities when used to analyze cyclic peptide therapeutics, where it is able to aid in rapid soft spot identification. Additionally, HRIM-MS enhances the analysis of biopharmaceuticals by efficiently filtering out interfering compounds for direct analysis within a complex mixture. These findings demonstrate the utility and versatility of HRIM-MS, showcasing its capacity to enhance and streamline pharmaceutical characterization workflows.
The efficacy of pharmaceutical drugs brought to market continues to increase as drugs have become more effective, selective, and potent. Continuous improvement of analytical characterization tools is needed to keep up with the increasing complexity of these drugs and to ensure the safety risks associated with these life saving products remain low. High-resolution ion mobility–mass spectrometry (HRIM-MS) is an analysis technique where ionized analytes are separated in the gas phase by their size and shape prior to mass analysis. HRIM-MS has been shown to improve the workflows used to analyze pharmaceutical products because it can be easily coupled with liquid chromatography (LC) to provide an additional dimension of separation. The time scales of LC, HRIM-MS, and quadrupole-time-of-flight tandem mass spectrometry (QTOF-MS/MS) neatly nest within each other to provide a complete answer.
Impurity Analysis of Lipid Nanoparticles
One of the challenges with therapeutic drug development is ensuring that the drug product is delivered to the intended target within the body. Storage prior to administration and normal biological processes within the body once administered can lead to undesired degradation of the drug product, reducing its efficacy. A method used to improve the stability and delivery of therapeutics is to encapsulate the drug product within a biocompatible material to protect it until it reaches the intended target.
One delivery platform that has gained success is lipid nanoparticles (LNPs), which are colloidal particles composed of lipids, typically arranged in a lipid bilayer structure, that are used to encapsulate hydrophobic or amphiphilic drugs within their core or lipid membrane. They are commonly used as drug delivery systems because of their biocompatibility, versatility, and ability to encapsulate a wide range of therapeutic agents.
LNPs were first approved by the U.S. Food and Drug Administration (FDA) to encapsulate small molecule anti-cancer drugs in 1995. LNPs have been successfully used to deliver messenger RNA (mRNA) as a therapeutic agent to prevent and treat various diseases, most recently approved by the FDA for use against Covid-19. The encapsulating LNPs protect the delicate nucleic acid from degradation after it is administered and allow cellular uptake and mRNA release (1).
Cationic or ionizable lipids are typically used as the primary structure of LNPs; however, formulations used for mRNA delivery typically contain other lipid types to improve nanoparticle properties such as particle stability, drug delivery efficacy, tolerability, and biodistribution (1).
Drug developers need the ability to confidently characterize the components of LNPs and identify any impurities introduced by the production process because these contaminants may affect payload delivery, particle stability, and efficacy. This is a challenge because of the complexity and isomeric nature of the lipids used in LNP designs. When using liquid chromatography–mass spectrometry (LC–MS) alone, interferences caused by coeluted peaks can make the analysis difficult and time consuming.
An example analysis of the ionizable lipid DLin-MC3-DMA, commonly used in LNP formulations, is shown in Figure 1. If LC–MS alone was used with a fast 1.5-min LC gradient to analyze the formulation mixture, the isotopic profile presented in Figure 1a would be recorded as the mass spectra of the DLin LC peak. If HRIM-MS is added to this workflow, without any changes to the chromatography, we find that a second species has coeluted with the DLin lipid, as shown in Figure 1b, that can be independently analyzed as it is separated in the gas phase.
FIGURE 1: Lipid nanoparticles analyzed by HRIM-MS system.
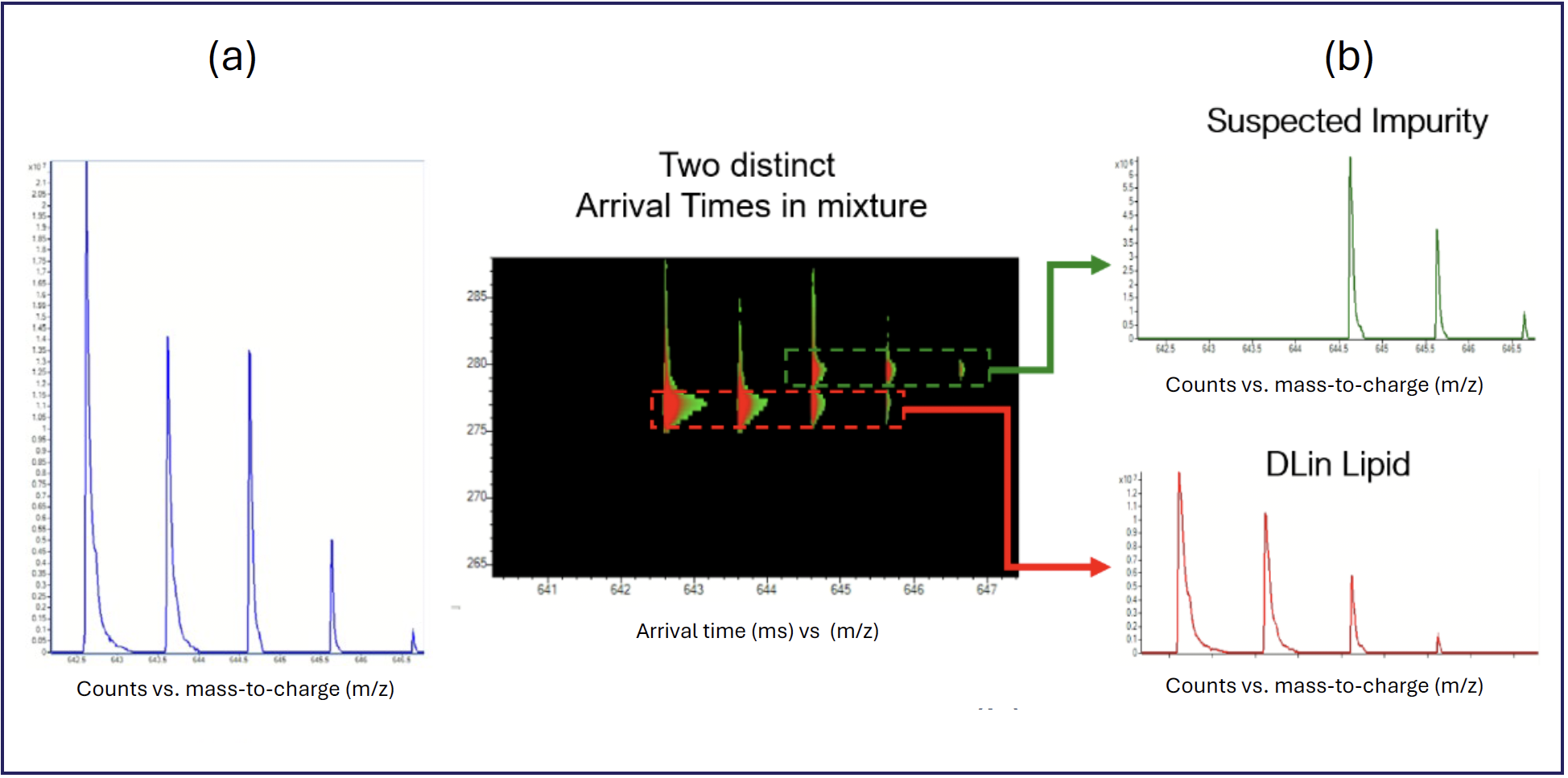
This fundamental gas phase separation presents the analyst with an unambiguous way to rapidly resolve two otherwise overlapping signals without reliance on long chromatography or sophisticated fragmentation techniques to detect and identify compounds. The use of HRIM-MS in this workflow results in higher analysis confidence because each analyte can be physically separated and analyzed independently.
Enhanced Cyclic Peptide Therapeutic Analysis
Peptide-based therapeutics comprise short chains of amino acids linked by an amide bond that are typically 500–5000 Da in molecular weight (2,3). Peptide therapeutics have gained increasing attention in recent years because of their specificity, potency, and relatively low toxicity compared to traditional small molecule drugs. Peptides can be designed to target specific molecular pathways or disease processes. As of May 2023, 114 peptides have been approved by regulatory authorities for therapeutic or diagnostic purposes (4).
Peptides can be used therapeutically to act on a variety of biological functions and were first used to regulate the hormone insulin to treat diabetes. Peptide therapeutics have been developed and approved to prevent and treat a variety of diseases including hypertension, certain types of cancer and osteoporosis (3).
Of the approved peptide therapeutic and diagnostic molecules on the market, 46% are classified as cyclic peptides, where the amino acid chain forms a closed ring structure instead of an open or linear structure. The cyclization of peptides improves the binding affinity and selectivity to the target, and can improve metabolic stability as cyclic peptides can be engineered to be resistant to undesired enzymatic degradation, because of their lack of a terminal end group (4).
Understanding how a cyclic peptide degrades or is metabolized, starting with the first site of hydrolysis, is critical to improving the stability and half-life of the drug product and, as a result, the therapeutics’ efficacy. This involves identifying the susceptible soft spot sites on the cyclic peptide ring, a process referred to as soft spot identification (SSID). Poor in vivo drug half-lives caused by undesired degradation is one of the main causes of failure in Phase I and Phase II trials (3).
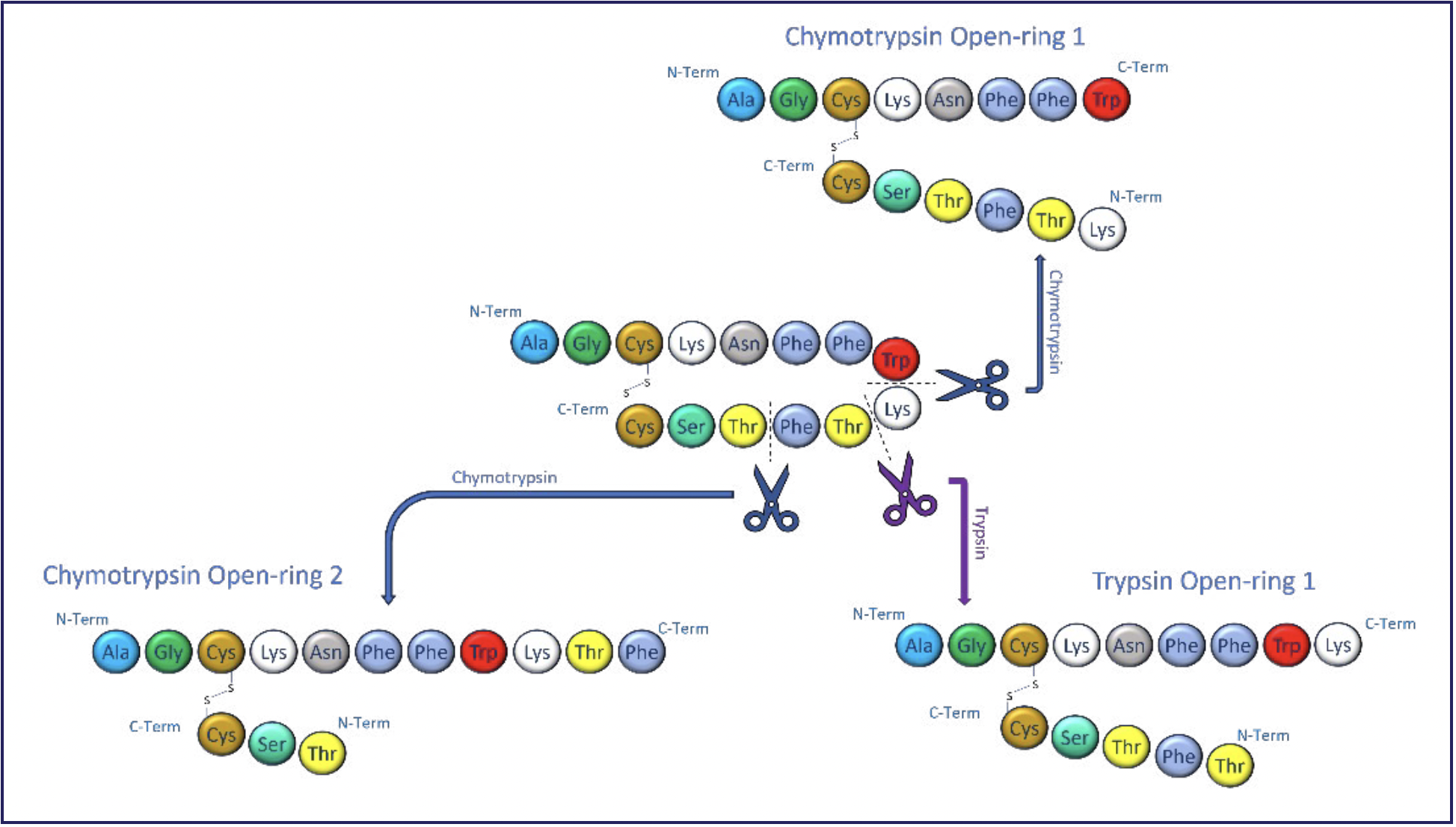
During hydrolysis, the ring structure of the cyclic peptide is opened to create a linear version of the peptide. Understanding and characterizing where this occurs on the cyclic peptide ring is crucial to improving drug stability as the opening sites can be engineered to be more or less resistant to hydrolysis, as required. SSID of cyclic peptides is challenging because a single hydrolysis event can open the ring at different locations on the peptide. Figure 2 graphically shows how the cyclic peptide somatostatin, a hormone regulator, is incubated with two enzymes that naturally occur in the body. This represents how this peptide could degrade in vivo after it was administered. Cleavage of the cyclic peptide at these soft spot sites produces three peptides with different structures but the same mass.
FIGURE 2: Digestion of somatostatin by various enzymes.
An analytical challenge arises when there are multiple soft spots on the peptide ring because the resulting linear peptides are isomeric and often unresolved chromatographically. Fragmentation techniques are needed to identify the site of the soft spot. When unresolved chromatographically, the resulting fragmentation spectra are chimeric because multiple forms of the peptide are fragmented simultaneously, leading to ambiguities in hydrolysis site assignment. Consequently, current methods to identify isomeric cleavage sites can require lengthy tagging or LC workflows.
In contrast, HRIM-MS with mobility aligned fragmentation (MAF) can be added to the LC–MS workflow to physically separate the isomeric peptides in the gas phase before fragmentation and MS2 analysis, thus eliminating the chimeric spectrum that would otherwise exist. Figure 3 shows mass and mobility spectra of a mixture of chymotrypsin digested somatostatin.
FIGURE 3: HRIM separation of closed and two open ring forms of somatostatin using an HRIM-MS system.
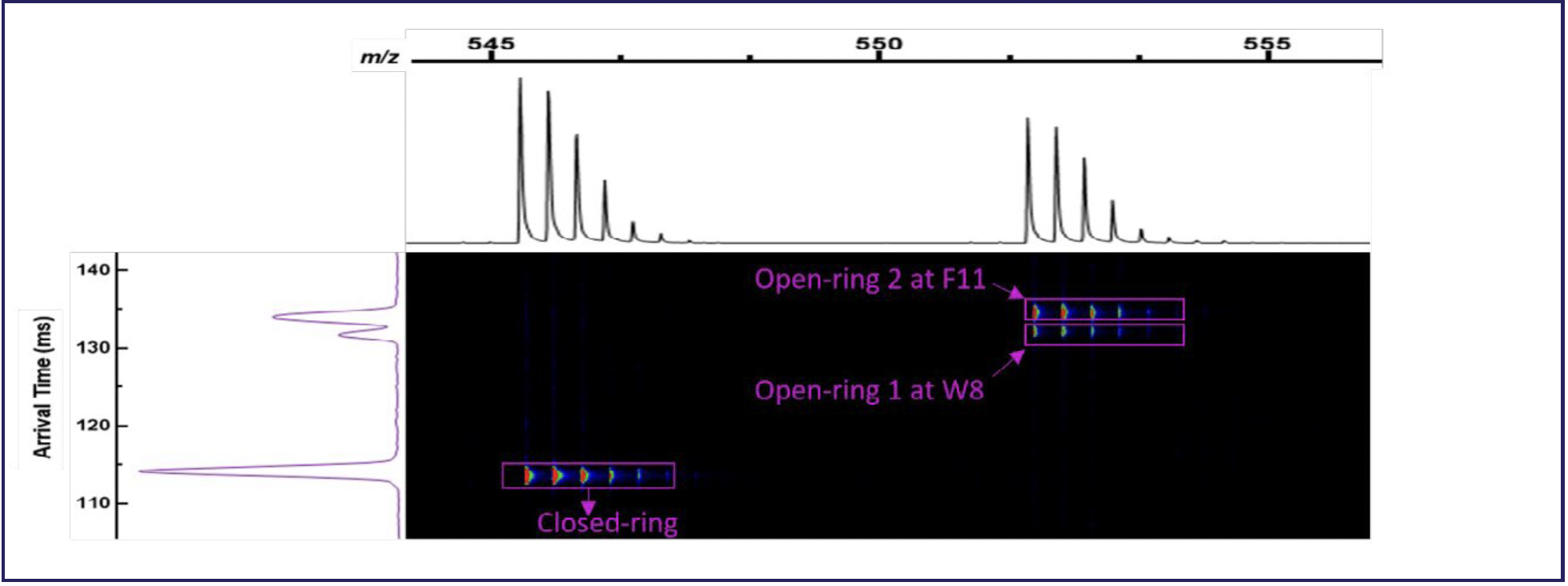
The m/z axis at the top of the figure shows two mass resolved isotopic profiles separated by a mass shift representing the close-ring form of the cyclic peptide and the digested open ring forms. As expected, no differentiation between the various possible forms of the open ring peptide can be made in the mass dimension, and they were not resolved chromatographically with a 10-min gradient. On the left side, the arrival time dimension shows three peaks: one indicating the closed ring form of somatostatin, which is mass resolved, and two near baseline resolved peaks at the mass of the open ring forms. The two resolved open ring peaks indicate the detection of two open ring forms of somatostatin where LC–MS alone previously revealed only one.
Identification of the location where the ring was opened is accomplished using MAF, where all ions are fragmented without mass isolation, with the difference in the arrival time from the HRIM-MS separation serving the role of traditional quadrupole mass filtering. Figure 4 shows the utility of MAF as fragmentation of the later arriving mobility peak gave a definitive identification of the open ring form 2 diagrammed previously in Figure 2.
FIGURE 4: Identification of open ring isoform by MAF.
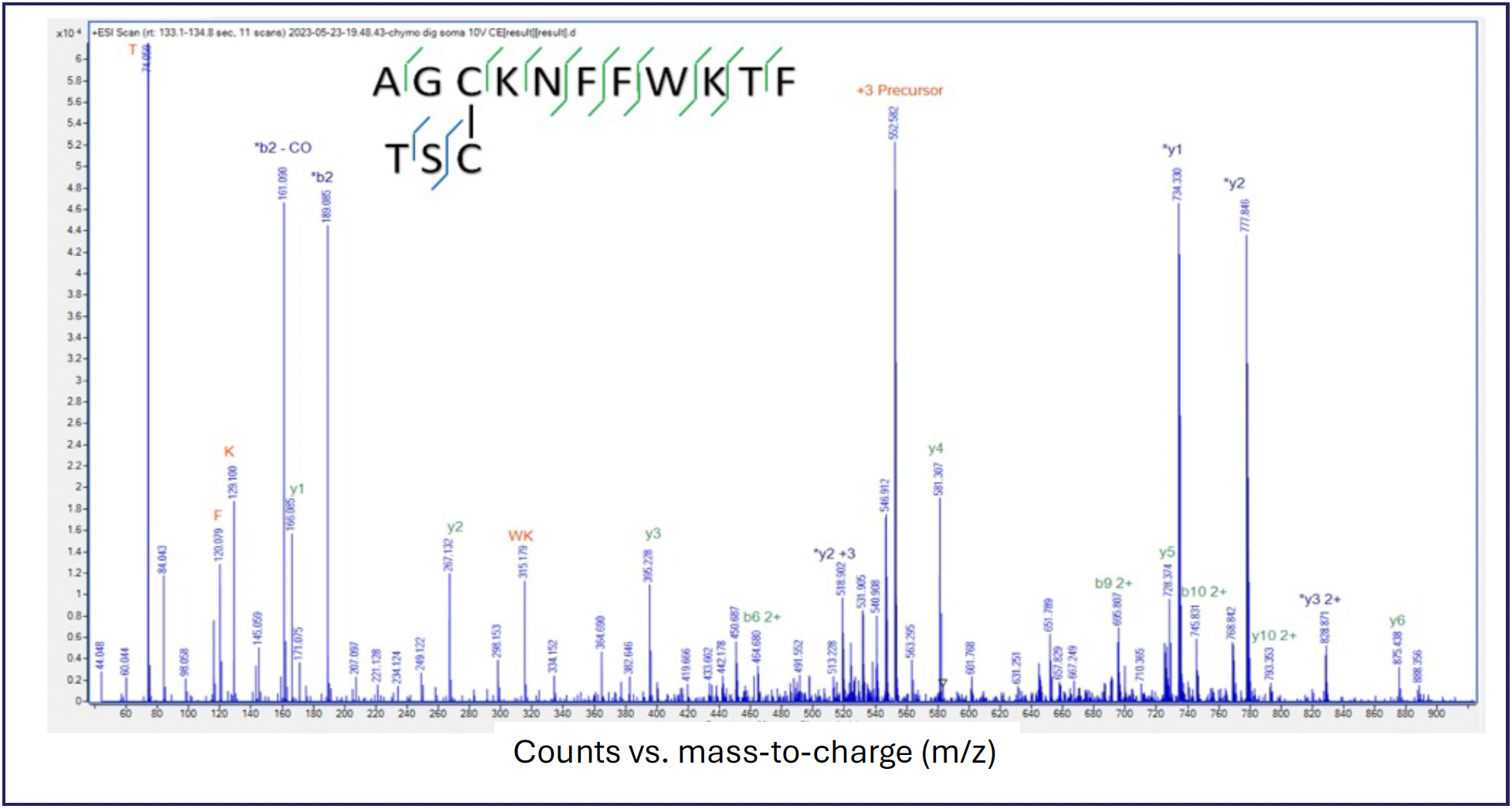
HRIM-MS has been shown to be a powerful technique for cyclic peptide soft spot analysis by adding a complimentary dimension of separation to an existing LC–MS workflow so that long LC run times, sophisticated MS2 spectral deconvolution, or complex tagging methods, are not needed to produce satisfactory results. The physical separation of the various open ring forms of the cyclic peptide in the gas phase prior to mass spectral analysis provides a simple and robust method to tackle this important analytical challenge.
Rapid Biopharmaceutical Impurity Analysis
Biopharmaceuticals, also known as biologically derived drugs, are complex molecules that are used to treat a range of disease types, and they offer unique advantages, such as high target specificity, potent biological activity, and the ability to modulate complex biological pathways. Examples of types of biopharmaceutical drugs include monoclonal antibodies, vaccines, recombinant proteins, and cell and gene therapies. These drugs are used in the treatment of various diseases, including cancer, autoimmune disorders, infectious diseases, genetic disorders, and metabolic diseases. The characterization of biologically derived drugs is challenging compared small molecule drugs as they are more complex and the manufacturing processes can introduce interfering compounds (5).
Biologically derived drugs must be purified from the cells used to produce them with control over the excipients required to achieve the goals of the drug product, as well as control over the impurities that must be removed to prevent adverse reactions. The resulting complex mixture may pose a challenge if using LC–MS alone.
HRIM-MS can be used to rapidly separate intact proteins from process residues so that impurities can be properly analyzed. Figure 5 shows a mixture of human serum albumin (HSA), myoglobin, ribonuclease A, and cytochrome C, analyzed using HRIM-MS size-exclusion chromatography (SEC) over a 3-min period.
FIGURE 5: Example of a protein mixture and resulting (a) HRIM filtered mass spectrum for each constituent as analyzed by HRIM-MS system: (b) myoglobin, (c) CytC, (d) HSA, and (e) RNAseA. Ordinate and abscissa axes labels for (b–e) are counts vs. mass-to-charge (m/z), respectively.
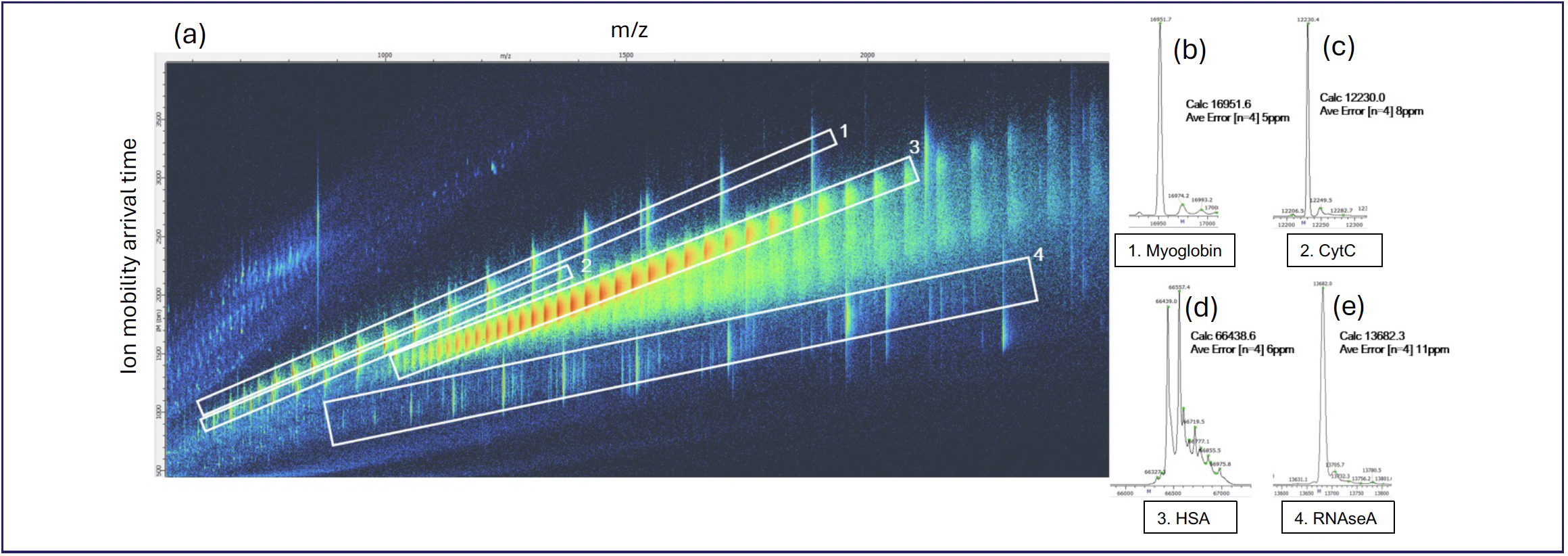
This example shows how HRIM-MS could be used to analyze a complex mixture of proteins and associated impurities quickly and confidently. The m/z axis shows the mass spectra of the mixture; because of the overlapping charge envelopes of each analyte present, accurate deconvolution will be a challenge with LC–MS alone, as these analytes were not resolved chromatographically. The analytes are resolved in the mobility dimension as each has a distinct “trendline” on the mass and mobility coordinate system. Using this trendline, the unique mass and mobility space can be individually selected and isolated to create a clean mass spectrum that can be accurately deconvoluted into the nominal mass of each analyte. Of particular interest is the RNAseA region (polygon #4) in Figure 5, which is an example of a low-level impurity that would be difficult to detect without the complementary HRIM-MS gas phase separation provided by this workflow. As shown here, HRIM-MS can be used as rapid screening tool for the detection of impurities inherent to the process of producing biological pharmaceuticals.
The three examples discussed show how HRIM-MS provides a distinct separation capability to optimize analytical operations throughout drug development. HRIM-MS can reveal crucial properties in increasingly complex medications that other methods leave unseen. These results indicate the relevance and versatility of using HRIM-MS to improve and simplify pharmaceutical characterization methods.
References
(1) Hou, X.; Zaks, T.; Langer, R.; et al. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. DOI: 10.1038/s41578-021-00358-0
(2) Barmen, P.; et al. Strategic Approaches to Improvise Peptide Drugs as Next Generation Therapeutics. Int. J. Pept. Res. Ther. 2023, 29, 61. DOI: 10.1007/s10989-023-10524-3
(3) Wang, L.; Wang, N.; Zhang, W.; et al. Therapeutic Peptides: Current Applications and Future Directions. Sig. Transduct. Target Ther. 2022, 7, 48. DOI: 10.1038/s41392-022-00904-4
(4) Costa, L.; Sousa, E.; Fernandes, C. Cyclic Peptides in Pipeline: What Future for These Great Molecules? Pharmaceuticals 2023, 16 (7), 996. DOI: 10.3390/ph16070996
(5) FDA – U.S. Food and Drug Administration. Biological Product Definitions. https://www.fda.gov/files/drugs/published/Biological-Product-Definitions.pdf (accessed 2024-05-02).
ABOUT THE AUTHOR
Gregory Webster is the Vice President of Product Development at MOBILion Systems, Inc. Direct correspondence to: gregory.webster@mobilionsystems.com

Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Characterizing Polyamides Using Reversed-Phase Liquid Chromatography
May 5th 2025Polyamides can be difficult to characterize, despite their use in various aspects of everyday life. Vrije Universiteit Amsterdam researchers hoped to address this using a reversed-phase liquid chromatography (RPLC)-based approach.
New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






