LC–MS/MS Array Created to Help Prognoses for Lung Cancer
In a recent study led by scientists at Maastricht University Medical Center in Maastricht, the Netherlands, a new LC–MS/MS assay was created to test new small molecular inhibitors (SMIs) and their effectiveness in prognosing lung cancer. Their findings were later published in the Journal of Chromatography B (1). SMIs are small organic molecules designed to bind to specific target proteins within cells, thereby modulating their metabolic activity.
lung cancer | Image Credit: © utah51 - stock.adobe.com
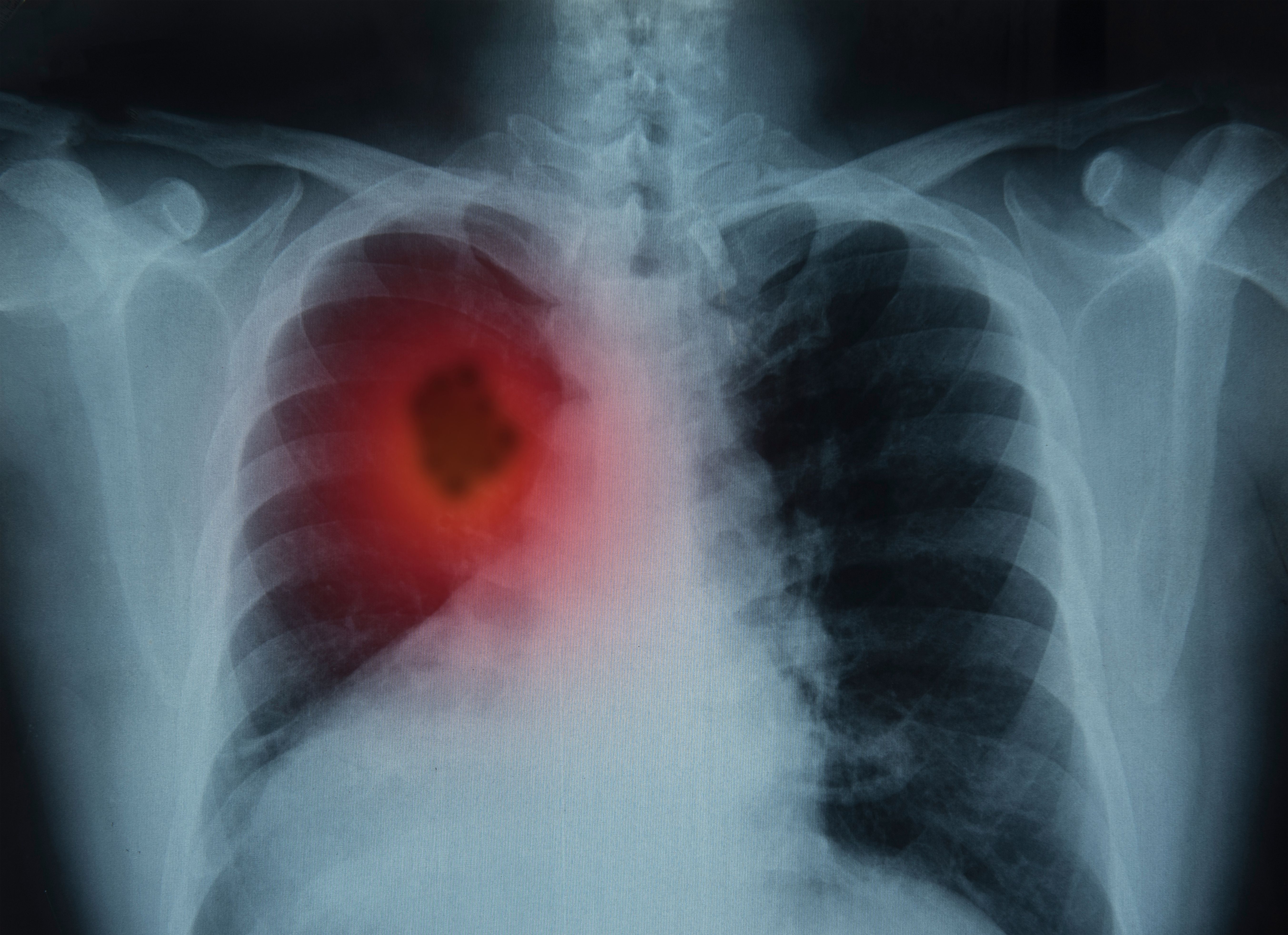
Lung cancer is the second most common cancer in the United States. For 2024, the American Cancer Society estimates approximately 234,580 new cases of lung cancer (116,310 in men and 118,270 in women) and 125,070 deaths from lung cancer (65,790 in men and 59,280 in women) (2). Of these cases, most will consist of non-small cell lung cancer (NSCLC), which accounts for roughly 85% of all lung cancer cases worldwide and causes approximately 1.6 million deaths globally each year. Unfortunately, patients with advanced NSCLC have poor prognoses. To rectify this, multiple approaches, such as targeted small molecular inhibitor (SMI) therapy, have been used to improve prognosis for these patients. Two new SMIs, sotorasib and adagrasib, are believed to be useful against targeting Kirsten rat sarcoma (K-RAS) p.G12C mutations, to the point where they have gained regulatory (FDA) approval.
To support pharmokinetic research and clinical decision making with these findings, a simple and accurate liquid chromatography–tandem mass spectrometry (LC–MS/MS) method was developed for multiplexed quantification of adagrasib and sotorasib. Further, this method was co-validated to perform the quantification for several targeted cancer therapies known as tyrosine kinase inhibitors (TKIs), such as brigatinib, lorlatinib, pralsetinib, and selpercatinib. Methanol was used for single-step protein precipitation, and chromatographic separation was performed with an Acquity® HSS C18 ultrahigh-pressure liquid chromatography (UHPLC) column. During this process, the elution gradient consisted of ammonium formate 0.1% v/v in water and acetonitrile.
In K2-EDTA plasma, adagrasib proved stable for at least seven days, at room temperature and 4 ºC, and for at least 3 months at -80 ºC. As for sotorasib, it was found to be stable for at least three days at room temperature, seven days at 4 ºC, and at least 3 months at -80 ºC. This method was validated over linear ranges of 80–4000 ng/mL for adagrasbi and 25–2500 ng/mL for sotorasib. With these findings, the method’s selectivity, carryover, precision, and accuracy met the requirements described in the most recent International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) M10 guidance at the time. The method can be combined with previously reported methods for various other SMIs, and this approach can assist quantification of the currently most-used SMIs in the Netherlands using a single-assay set-up. There is more work to be done here, but this assay has been deemed well-equipped for determining plasma concentrations in clinical practice.
References
(1) Kruithof, P. D.; de Beer, Y. M.; Gulikers, J. L.; et al. Validated Extended Multiplexed LC–MS/MS Assay for the Quantification of Adagrasib and Sotorasib in Human Plasma, Together with Four Additional SMIs. J. Chromatogr. B 2023, 1231, 123918. DOI: 10.1016/j.jchromb.2023.123918
(2) Key Statistics for Lung Cancer, American Cancer Society, Inc. Home Page. https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (accessed 2024-4-16)
Accelerating Monoclonal Antibody Quality Control: The Role of LC–MS in Upstream Bioprocessing
This study highlights the promising potential of LC–MS as a powerful tool for mAb quality control within the context of upstream processing.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Prioritizing Non-Target Screening in LC–HRMS Environmental Sample Analysis
April 28th 2025When analyzing samples using liquid chromatography–high-resolution mass spectrometry, there are various ways the processes can be improved. Researchers created new methods for prioritizing these strategies.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





