Ionization Efficiency for Environmentally Relevant Compounds Using Atmospheric Pressure Photoionization Versus Electrospray Ionization
Special Issues
In this study, atmospheric pressure photoionization (APPI) is compared to the default ionization method, electrospray ionization (ESI), for solution-phase samples. These mass spectrometry methods are compared and optimized relative to artificial wastewater for the detection and quantitation of pharmaceuticals frequently found as environmental contaminants.
For solution-phase samples, the world of mass spectrometry defaults to electrospray ionization (ESI). ESI is used for the analysis of a broad variety of compounds, ranging from polar to moderately nonpolar. However, ESI possesses limitations that prevent the ionization of certain analytes-particularly nonpolar compounds. This study aims to compare the ionization efficiency of complementary ionization techniques, and demonstrate that multiple methods can improve the analytical results with respect to limits of detection and matrix tolerance. Atmospheric pressure photoionization (APPI) is an ionization method that complements ESI, excelling in the analysis of nonpolar and moderately polar analytes. For this study, we optimized methods using APPI and ESI for the detection and quantitation of pharmaceuticals frequently detected in the environment, including antibiotics, beta-blockers, and selective-serotonin reuptake inhibitors, and tested their matrix tolerance relative to artificial wastewater. While most of these compounds ionized preferentially by ESI, some performed significantly better using APPI.
Every day in the world of analytical chemistry, we strive to improve our analytical methods to achieve lower limits of detection, have broader dynamic ranges, or tolerate greater interference, for example. Along with the need for improved methods of quantitation, there is pressure on the field of mass spectrometry (MS) to be able to successfully detect multitudes of analytes of interest from single injections, which poses the question, How do we improve the detection of analytes? However, sometimes using a single injection reduces performance by only using a single ionization method. When you can optimize not only your separation, but also your ionization method, your method may truly improve. The basic principle of MS is to ionize molecules under study into gaseous ions, separate these ions based on their mass to charge (m/z) ratio, and detect them (1). Today's commercial instruments are capable of transferring 97–99% of ions successfully from the source to the detector, so the greatest improvements in detection and methods today are focused on the source. The ultimate question then becomes, When ionizing a sample, will the use of complementary ionization techniques improve the figures of merit of the individual analytes?
ESI and APPI: Complementary Ionization Techniques
By choosing the appropriate ionization source, liquid chromatography–tandem mass spectrometry (LC–MS/MS) can be used for the detection of trace levels of contaminants like antibiotics and endocrine disruptor compounds (EDCs) from environmental samples. The electrospray ionization (ESI) source has been used as a powerful soft ionization technique for the analysis of a wide array of sample types, ranging from polar to nonpolar by MS, and can also be used for the analysis of thermolabile molecules of high molecular weight (2). Although ESI is popularly used for the analysis of environmental pollutants, it may not be able to ionize all contaminants efficiently. Certain contaminants are either poorly ionized, or not ionized at all. ESI is limited to analytes that are of low to high polarity, and moderate to high molecular weight (3).
Atmospheric pressure photoionization (APPI), introduced in 2000, is also a soft ionization technique. APPI was found to have success with the analysis of compounds with low to no polarity, and compounds of low to moderate molecular weight, but cannot be used on thermolabile compounds. These parameters are what make APPI and ESI complementary to each other (4). This opens new doors for studies already utilizing the ESI method, because APPI can be used for complementary analysis of compounds that may not be detected by ESI. Additionally, APPI has shown tolerance to matrix components beyond what ESI has, due to its ionization pathway (5). APPI can be used for the analysis of a wide range of compounds, including drugs, human endogenous compounds, lipids, natural compounds, pesticides, synthetic organics, and petroleum derivatives (6). There are very few research papers reporting comparative studies of ionization efficiencies in MS for the detection of antibiotics and EDCs (7–9).
A triple quadrupole mass analyzer was used in this study utilizing both the full scan mode-optimization and multiple reaction monitoring (MRM)-quantitation. Full scan mode can give qualitative analysis of a sample's composition under study, and MRM mode is a highly selective mass monitoring mode with a wider linear dynamic range, improved limit of quantitation (LOQ), increased sensitivity, and superior accuracy. The advantage of the MRM scan mode is improved signal-to-noise ratio due to removal of nonanalyte ions and isobaric precursors by monitoring fragments.
There are different MS acquisition parameters that affect the signal intensity of ions. The Agilent MassHunter Data Acquisition Software used in this study sets a default value for all acquisition parameters for each ionization source (see table S1 in the
supplemental information). There is a sheath gas flow chamber in the electrospray ionization source that is absent in the atmospheric pressure photoionization source. As a result, the sheath gas temperature and sheath gas flow rate parameters are present only for ESI, while APPI has an additional vaporizer parameter that is not present in ESI. Fragmentor voltage, collision energy, cell accelerator voltage, gas temperature, vaporizer, gas flow (L/min), nebulizer (psi), sheath gas temperature, and sheath gas flow rate were all optimized for each analyte prior to data acquisition in this study.
Analytes of Interest
The current global population is growing at the annual rate of 1.09%. This increase in population means that pharmaceuticals are continuing to be prescribed and consumed at an alarming rate. In 76 countries across the globe, antibiotic consumption as described in defined daily doses (DDD) increased by 65%-from 21.1 billion doses in 2000 to 34.8 billion doses in 2015-and the overall antibiotic consumption rate has increased by 39% (10). In addition to antibiotics, beta blockers and antidepressants are two classes of pharmaceuticals gaining popularity. Beta blockers are a class of drugs frequently used to treat hypertension, heart disease, and other cardiovascular events. Although the true nature of their efficacy has been questioned in certain studies, beta blockers are still highly prescribed, due to the diverse range of clinical symptoms they can successfully treat (11–12). Per the National Center for Health Statistics (NCHS) in 2017, the rate of antidepressant use in America has increased by 65% since 1999 (13).
Unfortunately, this increase in pharmaceutical use means more pharmaceutical waste is likely to end up in the environment. Although there is an urgency to know the exact harm this excess will cause, the priority is to harness the ability to detect as many pharmaceuticals in environmental samples as possible. This will then allow for proper removal techniques to be employed before the harmful substances have a chance to further contaminate the environment (14).
Low Concentrations, Large Impact
Numerous studies have shown that some pharmaceuticals are not completely removed during the wastewater treatment, and ultimately enter the environment in low concentrations. The adverse effects of pharmaceuticals entering the environment in low concentrations are antibiotic resistance, genotoxicity, acute or chronic toxicity, and endocrine disruption (15). Antibiotics and EDCs are emerging pollutants detected throughout the world, yet they remain unregulated by the United States Environmental Protection Agency (EPA) (16).
Sir Alexander Fleming, the British bacteriologist, discovered penicillin in 1928 from the fungus Penicillium notatum, and what followed was an era of novel antibiotics derived from microorganisms and antibiotic synthesis (17). The ability of antibiotics to eradicate a wide range of bacterial infections led to their increased use over time. Unfortunately, bacteria have developed mechanisms to combat the actions of antibiotics. Thus, the overprescribing of antibiotics, along with a lack of patient knowledge regarding the importance of correct antibiotic administration, has become an insidious issue that is known as antibiotic resistance. Antibiotic resistance arises as microorganisms develop the ability to survive the action of antibiotics, meaning that when antibiotic resistant bacteria infect animals and humans, the antibiotic regimen that would normally eradicate the bacteria becomes useless. This is the reason being able to successfully detect antibiotics from wastewater samples is so important. In this study, five different classes of antibiotics were used: beta-lactams, macrolides, nitroimidazoles, sulfonamides, and tetracyclines.
Antibiotics can either be bacteriostatic, which means they prevent the growth of bacteria, or they can be bactericidal, meaning they actively kill bacteria. However, antibiotics' mechanism of action is more important when considering treatment options. Beta-lactams inhibit the biosynthesis of bacterial cell walls by making penicillin-binding proteins unavailable for new peptidoglycan synthesis, which causes the lysing of bacteria. The beta-lactams used in this study are ampicillin, ceftriaxone, cephalexin, and penicillin G. Macrolides inhibit protein synthesis during translocation in bacteria by dissociating peptidyl-tRNA from the middle of the 23S rRNA of the ribosome's 50S subunit, causing early detachment of unfinished peptide chains. The macrolides used in this study are erythromycin and tylosin, an antibiotic popularly used in farm animals. Tetracyclines prevent the attachment of aminoacyl t-RNA to the A site in bacterial ribosomes by acting on the 16S rRNA of the 30S subunit inhibiting protein synthesis. Oxytetracycline and tetracycline were the tetracyclines used in this study. Sulfonamides prevent the multiplication and growth of bacteria by inhibiting certain steps in the metabolism of folic acid. Sulfamethoxazole and trimethoprim were the sulfonamides used in this study. Nitroimidazole antibiotics inhibit nucleic acid synthesis that occurs in bacterial cells by disruption of the DNA in microorganisms. Metronidazole and 1,2 dimethyl-5-nitroimidazole were the nitroimidazoles used in this study (18).
EDCs are natural compounds or synthetic chemicals that mimic natural hormones in the body and interfere with the action of the natural hormones (19). These compounds most profoundly cause adverse effects on reproduction, developmental, neural and immune systems of human beings and animals. Research suggests that EDCs reduce fertility and the increase the risk of cancer, diabetes, obesity, and endometriosis (20). Among various EDCs, beta-blockers (acebutolol, atenolol, metoprolol and propranolol) and SSRI antidepressants (citalopram, paroxetine and venlafaxine) were used in this study.
Make or Break for Successful Analysis: Matrix Effects and Wastewater
Properly dealing with impurities is a necessary complication in every field of research. In MS, the problem with matrix is variability in ionization efficiency of analytes of interest as coeluted species serve to either enhance or inhibit the ionization process for an analyte. This issue becomes increasingly problematic when trying to discern analytes of interest from wastewater. Water that is obtained as a byproduct of agricultural, industrial, domestic, and commercial activity is termed wastewater. Wastewater contains nutrients such as calcium, iron, nitrogen, phosphorus, potassium, and components such as fats, sugars, and proteins. Synthetic wastewater was made to mimic the wastewater from the influent of a typical wastewater treatment plant with its composition designed to imitate the dissolved inorganic solids and dissolved organic solids of real wastewater. The synthetic wastewater prepared in this study was from H. E. Gray (2012) (21).
APPI has been found to be less susceptible to matrix effects compared to ESI. This is likely due to the fact that APPI is more selective in ionization, because the photon emitter krypton lamp at 10.6 eV can ionize analytes, but not the matrix component, meaning that the difference in how a sample is ionized can be the difference between more or less matrix interference (22). Using APPI involves the ejection of an electron from the analyte molecule to produce the gaseous radical cation (23). It is also possible, however, that the matrix component can act as a dopant and ionize sample components with high ionization energy through electron transfer leading to signal enhancement.
Although matrix effects cannot be removed completely, they can be minimized by optimizing the sample preparation procedure and LC–MS parameters. Solid-phase extraction (SPE) with an appropriate sorbent can reduce the matrix effect by eliminating interfering matrices. The formula for calculation of the matrix effect is:
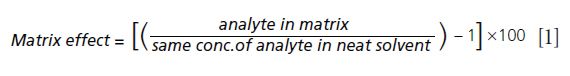
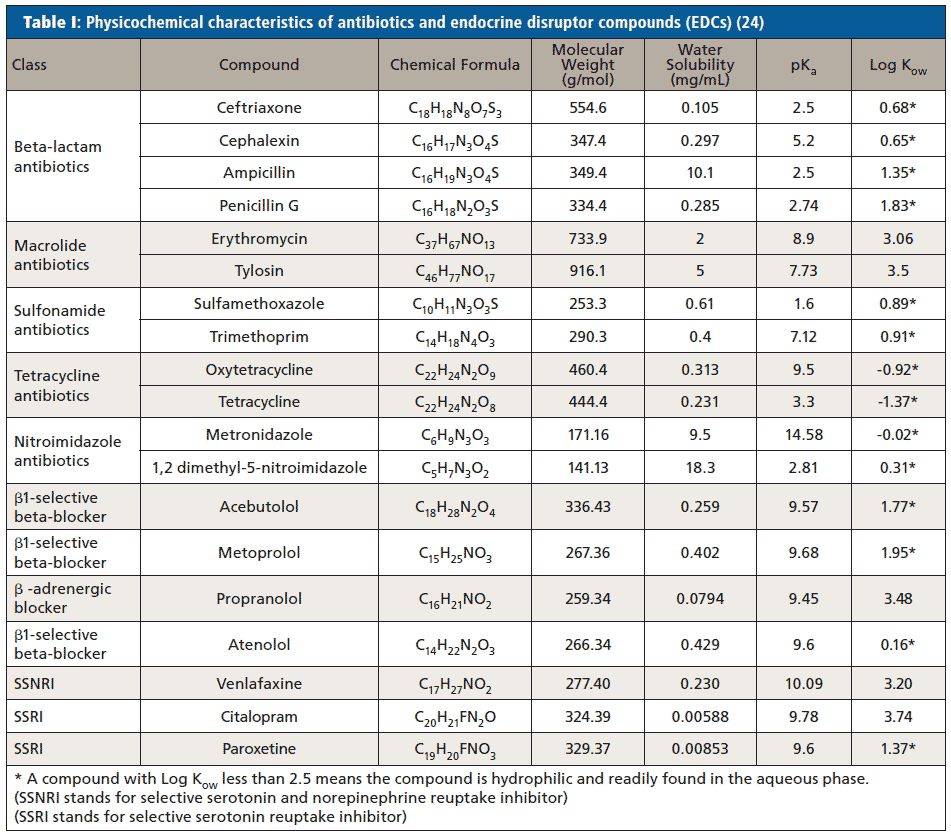
Physicochemical characteristics of antibiotics and EDCs help to determine the environmental fate of these compounds. Table I contains important physicochemical characteristics of antibiotics and EDCs under study including solubility, pKa, and log Kow. A compound with a log Kow value <2.5 is hydrophilic and readily found in the aqueous phase.
Methods
Specific Analytes Used
A total of 12 antibiotics and 7 EDCs were analyzed in this study. Ceftriaxone sodium salt hemi(heptahydrate) and erythromycin were purchased from Acros Organics with a purity of >98%. Propranolol hydrochloride (99%), metronidazole (99%), acebutolol hydrochloride, metoprolol tartrate (98+%), tetracycline hydrochloride (96%), and oxytetracycline hydrochloride were purchased from Alfa Aesar. TCI Co. was the main supplier of chemicals: cephalexin monohydrate (>98%), sulfamethoxazole (>98%), penicillin G potassium salt (>98%), atenolol (98%), venlafaxine hydrochloride (>98%), trimethoprim (>98%), citalopram hydrobromide (>98%), and 1,2 dimethyl-5-nitroimidazole. Ampicillin sodium salt was procured from Affymetrix Inc. Tylosin tartrate (95+%) and paroxetine hydrochloride (98+%) were bought from Ark Pharm Inc. All antibiotics and EDCs were used without further purification. All the solvents used in the analysis are of HPLC grade and purchased from Fischer Chemical. Potassium phosphate monobasic (99.8%) was purchased from EK Industries Inc. Sodium acetate trihydrate (100.7%), magnesium sulfate heptahydrate (99.9%), ammonium chloride (99.7%), and calcium chloride dihydrate (99.9%) were bought from Fischer Scientific. All solvents and chemicals were used without further purification.
Optimized Parameters
The parameters were optimized for MS as follows: 1.00 ppm sample of each analyte was analyzed for the selection of the precursor ion, optimization of fragmentor voltage, optimization of cell accelerator voltage, optimization of gas temperature, optimization of gas flow rate, and optimization of collision energy. All the ions formed were analyzed for intensity. Ions with m/z values equal to and greater than the molecular weight of the analyte were considered to determine the precursor ion. See Figure 1 for the flowchart of the optimization strategy.
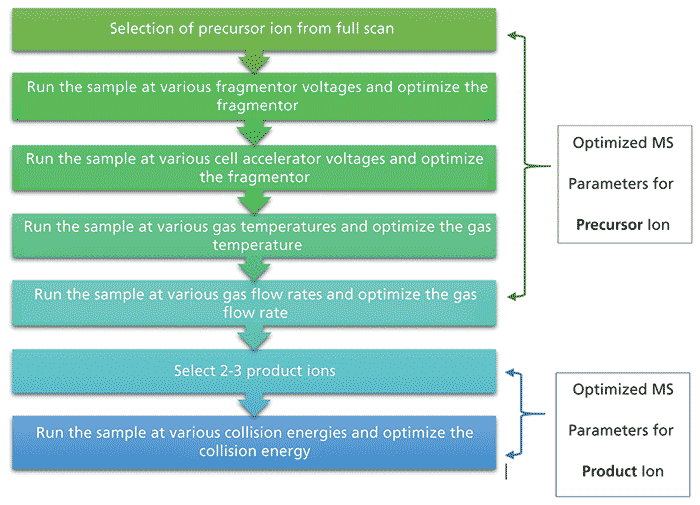
Figure 1: Flowchart of the ionization optimization strategy used in this study.
A calibration curve for each analyte was obtained by the internal calibration method using the optimized MS parameters. Calibration was performed in the range of 1.00 ppt to 10.0 ppm for each analyte under study. Each of the standard solutions for antibiotics was spiked with the mixture of internal standards of antibiotics (azithromycin d3, cephalexin d5, ciprofloxacin d8, penicillin G d5, sulfamethoxazole d4, and trimethoprim d3) to produce a final concentration of 100 ppb of each internal standard. Each of the standard solutions for EDCs was spiked with the mixture of internal standards of EDCs (metoprolol d7 and paroxetine d6) to produce a final concentration of 10.0 ppb of each internal standard.
A synthetic wastewater matrix solution was prepared by dissolving potassium phosphate monobasic, sodium acetate trihydrate, magnesium sulfate heptahydrate, ammonium chloride, and calcium chloride dihydrate in MilliQ water. The concentration and quantity of reagents used for synthetic wastewater matrix preparation is given in
supplemental tableS2.
Analysis of the antibiotics and EDCs was performed using an Agilent Technologies 1290-6460 Triple Quadrupole LC–MS/MS instrument using two ionization sources: an Agilent Jet Spray ESI source and an Agilent APPI source operated in positive mode. Full scan mode was used for the optimization of MS parameters, and MRM mode was used for calibration and analysis of wastewater. Data interpretation was performed using Agilent's MassHunter Workstation Software. HPLC parameters for analysis are given in
supplemental tableS3.
Calibration was performed in the range of 1.00 ppb to 10.0 ppm in matrix for all the analytes under study. Internal standards were added as described previously.
A setup of Waters Oasis Prime HLB cartridges and a SPE vacuum manifold was used for off-line SPE to extract antibiotics and EDCs from the synthetic wastewater matrix calibration sample. Waters Oasis Prime HLB cartridges, 1 mL barrel syringe with 30 mg universal polymeric reversed-phase sorbent, were employed. SPE pretreatment was performed by washing the column with 2 mL of HPLC-grade methanol, 2 mL of Millipore deionized water, and 2 mL of Millipore deionized water at pH 2 under gravity. Then the samples were loaded on the column under vacuum at 10–20 mL/min rate.
Washing and Elution Step for Antibiotics Sample
After loading the sample on the column, the cartridge was washed with 2 mL Millipore deionized water for the antibiotics sample. The column was then washed first with 2 mL of methanol, and then with 1 mL of methanol: acetone (1:1) under gravity, and collected and combined in test tubes.
Washing and Elution Step for EDCs Sample
EDCs sample cartridges were washed with 1 mL of methanol:water (5:95) solvent. After the first washing step, the column was dried for 15–30 min under vacuum. The column was washed first with 1 mL of ethyl acetate:methanol (9:1) under gravity (this eluate was collected in a test tube labeled as fraction 1), then washed with 1 mL of 5% methanol:2% acetic acid in water, then with 1 mL of 5% methanol:2% NH4OH in water under vacuum, and dried for 10–15 min under vacuum. After drying, the column was eluted with 1 mL of 2% NH4OH in methanol, and combined with eluate present in the test tubes labeled as fraction 1.
Wastewater Sample
Wastewater samples were collected from the influent of the aeration treatment at the Environmental Resources Training Center (ERTC), a training center for drinking water and wastewater treatment at Southern Illinois University Edwardsville (SIUE). The samples were analyzed for the presence of antibiotics and EDCs to demonstrate the effectiveness of the method development on real wastewater samples.
The wastewater samples were sequentially filtered through VWR 417 (40 µm) filter paper, then through VWR 696 (1.2 µm) glass microfiber filter paper, and then through an Ahlstrom 193 (0.7 µm) microfiber glass filter. The filtrate was separated into 6 bottles each with 250 mL of filtrate, 3 samples for analysis of antibiotics and 3 for EDCs. All samples were spiked with appropriate internal standards as described previously.
Samples were adjusted to pH 3.0 using 6.0 M sulfuric acid before performing the SPE. Waters Oasis Prime HLB cartridges (6 mL, 200 mg universal polymeric reversed-phase sorbent) were used for wastewater sample analyte extraction. The SPE method for wastewater was identical to the synthetic matrix sample preparation except the quantity of reagent solvents used was five times greater due to the increased volume and cartridge bed mass.
Results and Discussion
The optimized MS parameters for the precursor ion of antibiotics for ESI as an ionization source can be found in
supplemental tablesS4 and S5, while parameters for APPI are shown in S6 and S7. Ions with the highest intensity peak with a m/z equal to or greater than the molecular weight of the analyte were selected as potential precursor ions and MS parameters were optimized using these ions.
The optimized MS parameters for the product ion of antibiotics for ESI as an ionization source can be found in
supplemental tablesS8 and S9, while parameters for APPI are shown in S10 and S11. At most, three ions with m/z less than the molecular weight of the analyte and with the highest ion abundance were optimized to determine the optimized collision energy of the product ions.
The regression equations for antibiotics and EDCs without matrix were selected such that they were equivalent for calibration curve performed with and without matrix. The calibration curve correlation coefficient (R2) criteria was established as higher than 0.99 for all the antibiotics and EDCs without matrix using ESI, shown in
supplemental tablesS12 and S13. Ampicillin, ceftriaxone, cephalexin, sulfamethoxazole, oxytetracycline, tetracycline, and metronidazole have limits of detection (LODs) <1 ppt using ESI and sulfamethoxazole has the highest LOQ among the antibiotics analyzed (226.1 ppb). All standard calibration curves are shown in
supplemental tableS20. Paroxetine, propranolol, and venlafaxine have a LOD <1 ppt using ESI, and acebutolol has the highest LOQ(14.81 ppb).
Ampicillin, cephalexin, penicillin G, trimethoprim, and tetracycline have LOD values <1 ppt using APPI as the ionization, and tylosin has the highest LOQ among the antibiotics analyzed (3,401 ppb), as shown in
supplemental tableS14. Citalopram, paroxetine, and propranolol have LODs <1 ppt using APPI as the ionization source and venlafaxine has the highest LOQ (3,788 ppb) as shown in
supplemental tableS15.
The efficiency of both ionization sources was determined by comparing the LOQ for each of the pharmaceuticals obtained using ESI and APPI. Limits of quantitation <1 ppt in both the ionization sources means the most efficient ionization source could not be determined. Any compounds that are thermolabile will degrade using APPI, meaning the compound will not be detected by APPI, and ESI was the ionization source that was used. Erythromycin, tylosin, and metronidazole ionized efficiently by ESI based on the comparative LOQ result. This hypothesized to be due to the pKa of each compound (Table I) being greater that the pH of the mobile phase (3.80) used in the analysis of antibiotics which allows it to protonate easily. Acebutolol, atenolol, metoprolol, and venlafaxine were ionized efficiently by ESI based on the comparative LOQ results obtained from the calibration curve performed with and without matrix. A complete breakdown of this analysis can be found in Tables II and III.
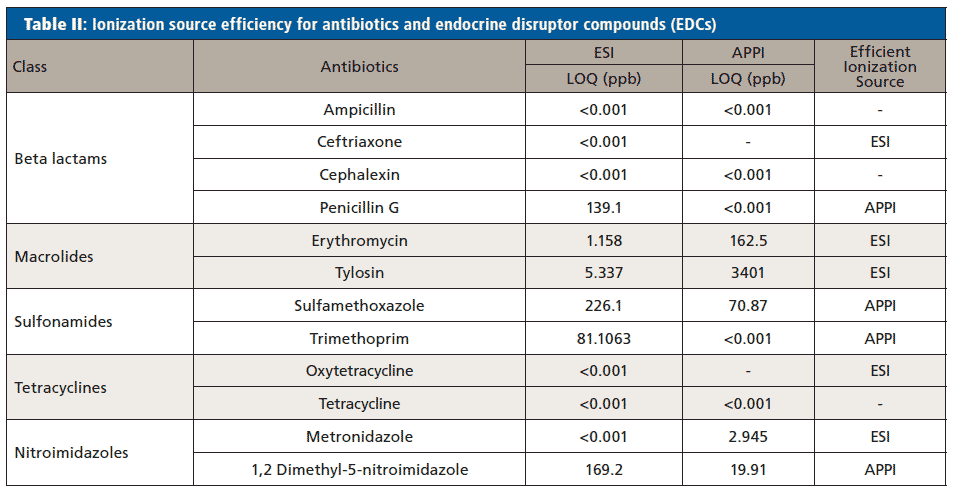
Penicillin G, sulfamethoxazole, and 1,2 dimethyl-5-nitroimidazole have lower LOQs when ionized by APPI, so APPI is the preferred ionization source for these analytes. The pKa values of these compounds (Table I) are less than the pH of the mobile phase (3.80) leading them not to be protonated in solution. In addition, sulfamethoxazole and 1,2 dimethyl-5-nitroimidazole each have a high degree of conjugation in their structures facilitating the absorption of photons and molecular radical ion formation (M+•) (24). This specific trend was not observed in the case of trimethoprim indicating that some other preferred ion formation pathway must be present. Citalopram also has a higher degree of conjugation in its structure which facilitates the absorption of photons and molecular radical ion formation (M+•) making the APPI source highly efficient for the analysis of these compounds.
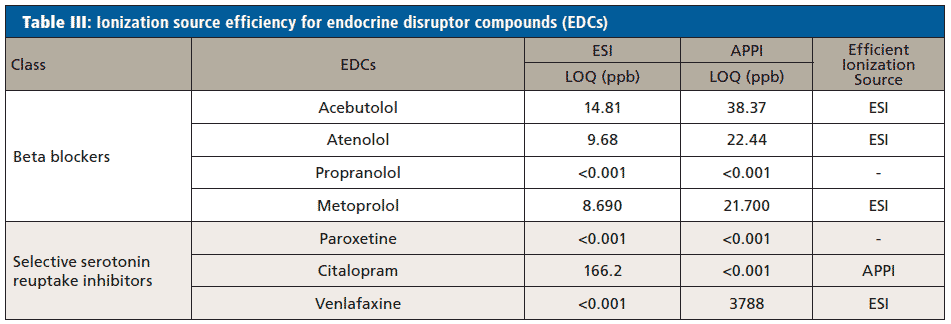
Two-way paired t-tests were conducted at a significance level (α) of 0.05 on the data of the calibration curve performed with an artificial matrix and without a matrix.
Supplemental tableS16 provides the p-values of the test for both the ESI and APPI ionization sources for analysis of antibiotics and shows that all population means are equal, therefore there is no significant difference between data obtained with or without a matrix.
supplemental tableS17 shows the population means are also equal between the data obtained with and without artificial matrix.
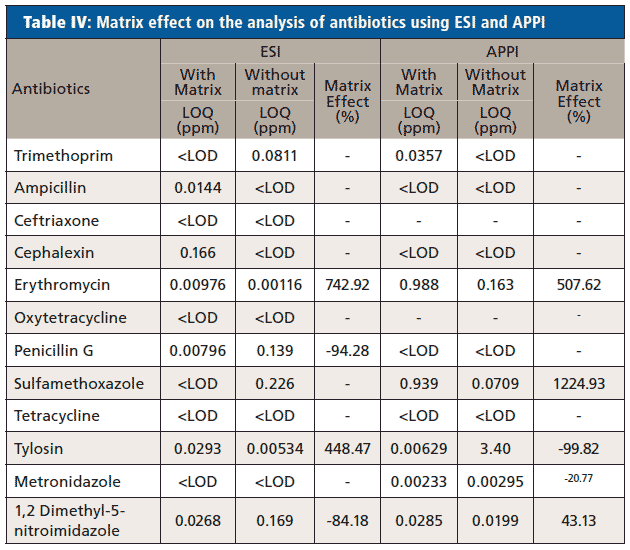
The matrix effects on antibiotics and EDCs using ESI and APPI was calculated using the limit of quantitation (LOQ) obtained from calibration curves performed with and without the matrix. The trends can be seen in Tables IV and V.
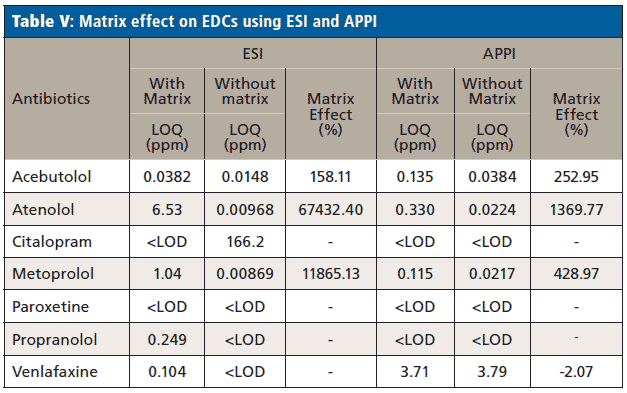
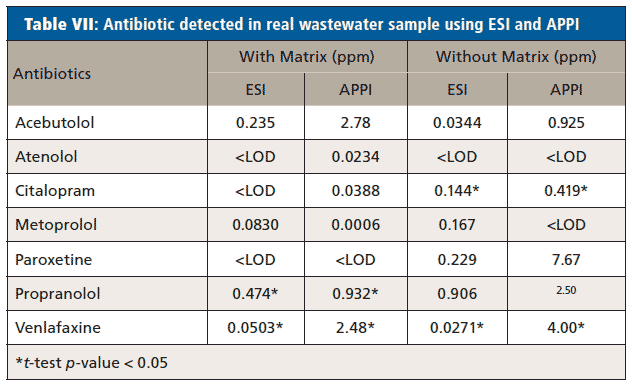
Real wastewater samples from the ERTC were analyzed for the detection of antibiotics and EDCs. Table VI shows the concentration of antibiotics in real wastewater samples and Table VII shows the EDCs calculated using the calibration curve equation obtained from calibration curve performed with and without synthetic matrix using ESI and APPI.
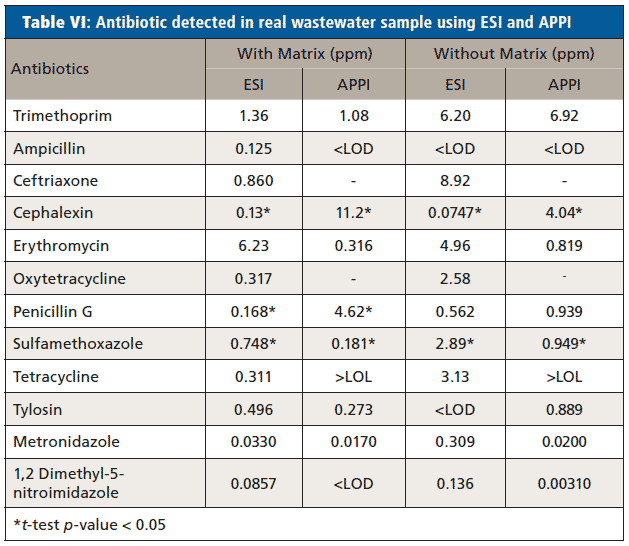
Paired t-tests were used to compare the concentration of antibiotics and EDCs in sample calculated using calibration curve performed with artificial matrix and without matrix. Two-way paired t-tests were conducted at a significance level (α) of 0.05 and the significance difference between the concentration of antibiotics and EDCs on the real wastewater sample collected from ERTC wastewater treatment plant using the equation obtained from calibration curve performed with artificial matrix and without a matrix were determined. Only three of the p-values shown in
supplemental tablesS18 and S19 demonstrated a statistical difference between using the matrix calibration curve versus the water curve illustrating that while important for some analytes, overall it had insignificant effect in this specific study.
Conclusion
There are few studies comparing the efficiencies of ionization sources for the analysis of pharmaceutical analytes. While many researchers are limited to using ESI as their only ionization source, the use of complementary ionization techniques produces better results for the quantitation of analytes, and should thus be considered for future studies. When purchasing an instrument costing $250,000 and up, the addition of a $25,000 additional ion source in order to improve analyte coverage in one's analysis should be viewed as nearly doubling the analytical capabilities of the instrument in terms of analyte coverage.
It was found that ESI is preferable for the analysis of pharmaceuticals such as antibiotics, beta-blockers, and SSRI antidepressants. However, ESI is not suitable for the ionization of all the pharmaceuticals with high sensitivity. APPI is an excellent complement to ESI; it is highly efficient in the ionization of analytes that ESI is unable to ionize.
There was no significant difference observed in the presence of a matrix at very low analyte concentrations. With higher concentrations of analytes, however, matrix effects should be taken into consideration when using these methods given the significant difference observed. Since the limit of quantitation of most of the analytes was <1 ppt, further study is needed to determine the ionization efficiency of ESI and APPI for these compounds by calibrating at lower concentrations.
By determining the ionization energy of analytes using the appropriate software, it can be predicted which compounds will ionize by APPI or ESI preferentially. This determination will aide in the analysis of other classes of environmental pollutants, including other groups of pharmaceuticals like statins and pesticides.
Using ESI and APPI as complementary ionization techniques yields a more complete picture of what compounds are present when analyzing in full scan mode and better quantitation of analytes when appropriately optimized. This information holds value because employing multiple ionization techniques is an easy fix that creates a cost-effective method for analyte detection that can improve the outcome of research in future studies.
Supplemental Information
The
supplemental tablescan be found by clicking the blue hyperlinks.
References
(1) S.D. Richardson, Anal. Chem. 80(12), 4373–4402 (2008).
(2) C.S. Ho, C.W.K. Lam, M.H.M. Chan, R.C.K. Cheung, L.K. Law, L.C.W. Lit, and H.L. Tai, Clin. Biochem. Rev. 24(1), 3–12 (2003).
(3) S. Banerjee and S. Mazumdar, Int. J. Anal. Chem. 2012 1–40 (2012).
(4) D.B. Robb and M.W. Blades, Anal. Chem. 78(23), 8162–8164 (2006).
(5) C.A. Wang, Chromatography 2(2), 1–3 (2015).
(6) I. Marchi, S. Rudaz, and J.L. Veuthey, Talanta 78(1), 1–18 (2009).
(7) A. Leinonen, T. Kuuranne, and R. Kostiainen, J. Mass Spectrom. 37(7), 693–698 (2002).
(8) A. Garcia-Ac, P.A. Segura, L. Viglino, C. Gagnon, and S. Sauvé, J. Mass Spectrom. 46(4), 383–390 (2011).
(9) A. Yamamoto, N. Kakutani, K. Yamamoto, T. Kamiura,and H. Miyakoda, Environ. Sci. Technol. 40(13), 4132–4137 (2006).
(10) E.Y. Klein, T.P. Van Boeckel, E.M. Martinez, S. Pant, S. Gandra, S.A. Levin, and R. Laxminarayan, Proc. Natl. Acad. Sci. 115(15), 3463–3470 (2018).
(11) C.S. Wiysonge, H.A. Bradley, J. Volmink, B.M. Mayosi, and L.H Opie, Cochrane Database of Systematic Reviews (2017).
(12) S. Akbar and M.S. Alorainy. Saudi Med. J. 35(11), 1307–1317 (2014).
(13) L.A. Pratt, D.J. Brody, and Q. Gu, National Center for Health Statistics 283, 1–8 (2017).
(14) A.B. Boxall, M.A. Rudd, B.W. Brooks, D.J. Caldwell, K. Choi, S. Hickmann, and G.T. Ankley, Environ. Health Perspect. 120(9), 1221–1229 (2012).
(15) M. Gros, D. Barcel, and S. Rodrs, J. Chromatogr. A 1248, 104–121 (2012).
(16) C.G. Daughton, Environ.Impact Assess.Review 24(7–8), 711–732 (2004).
(17) J.W. Bennett and K.T. Chung, Adv.Appl.Microbiol. 49, 163–184 (2001).
(18) G. Kapoor, S. Saigal, and A. Elongavan, J. Anaesthesiol. Clin Pharmacol. 33(3), 300–305 (2017). doi:10.4103/joacp.JOACP_349_15
(19) C. Monneret, C. R. Biol.340(9–10), 403–405 (2017).
(20) M. Street, S. Angelini, S. Bernasconi, E. Burgio, A. Cassio, C. Catellani, F. Cirillo, A. Deodati, E.Fabbrizi, V. Fanos, and G. Gargano, Int. J. Mol. Sci. 19(6), 1647–1691 (2018).
(21) H.E. Gray, Laboratory Methods for the Advancement of Wastewater Treatment Modeling. M.S. Theses and Dissertations (Comprehensive) [Online], (Wilfrid Laurier University, Waterloo, Ontario, Canada, 2012).
(22) W. Zhou, S. Yang, and P.G. Wang, Bioanalysis 9(23), 1839–1844 (2017).
(23) L. Silvestro, I. Tarcomnicu, and S. Rizea Savu, Tandem Mass Spectrometry- Molecular Characterization, Chapter: Matrix Effects in Mass Spectrometry Combined with Separation Methods - Comparison HPLC, GC and Discussion on Methods to Control these Effects (IntechOpen, London, United Kingdom, 2013).
(24) T.J. Kauppila, H. Kersten, and T. Benter, J. Am. Soc. Mass Spectrom. 26(6), 1036–1045 (2015).
Prakriya Shrestha, Katherine A. Maloof, Alayna Stephens, Clayton P. Donald, and Kevin R. Tucker are with the Department of Chemistry at Southern Illinois University Edwardsville, in Edwardsville, Illinois. Direct correspondence to: kevtuck@siue.edu

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.










