Hyaluronic Acid (Polysaccharides)
Wyatt Technology Application Note
Hyaluronic acid (HA) is an ubiquitous, very high molar mass polysaccharide that has been of particular importance in opthalmic surgery. HA acts as a molecular "shock-absorber" and stabilizer for cells and its visco-elastic properties are valuable for separating tissue and maintaining shape. It is a critical component in tissue lubrication and is believed to play a leading role in wound repair. Finally, HA's property of non-pyrogenicity makes it an ideal sheath for implants, whose presence might cause the body to suffer an immune response.
Among the many benefits high molecular weight HA hold are
- the maintenance of tissue space for surgery
- the protection of cells and tissue
- therapeutic effectiveness.
HA's therapeutic effectiveness depends critically on molecular weight: the higher the molecular weight, the longer its benefit. However, because of its visco-elastic properties, standards-based GPC analysis is inappropriate for characterizing HA. There exist no standards identical to HA and the desirability of altering experimental conditions renders conventional GPC/SEC impractical.
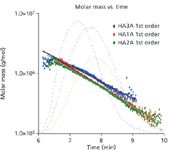
Figure 1: From the molar mass versus time plot, subtle differences can be seen among the samples.
Combining a DAWN with HPLC separation, however, provides an ideal platform for absolute characterization, since the lightscattering measurements do not depend on pump speed, polymer standards or molecular conformation.
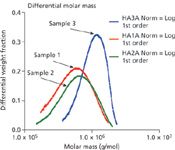
Figure 2: The differential molar mass distributions calculated by ASTRA immediately confirm the large differences among the three HA samples.
A DAWN was connected to a GPC/SEC line (100 mM NaN03 buffer, TSK-Gel G6000PW column, Optilab DSP refractometer, Waters 510 pump) and generated the data required to determine not only absolute molar mass, but also molecular size, for a variety of HA products.
Figure 1 shows the molar mass versus time (with the 90° light-scattering chromatograms in the background) for the three samples, ranging from approximately 2 million to less than 200K Daltons. Figure 2 illustrates how profoundly different the samples are by revealing their differential molar mass distributions. These indicate that the samples will behave in different ways when used medically, depending on the content of their high molecular weight HA.
Wyatt Technology Corp.,
6300 Hollister Ave.
Santa Barbara, California 93117, USA,
tel. +1 805 681 9009, fax +1 805 681 0123,
e-mail: info@wyatt.com, website: http://www.wyatt.com

New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










