From HPLC to UHPLC — And Back
Although ultrahigh-pressure liquid chromatography (UHPLC) method development is fast and cost efficient, many of the analytical methods used in quality control laboratories are still conventional high performance LC (HPLC) separations. Transferring these HPLC methods to UHPLC and validating them is a time-consuming and labour-intensive task. Nevertheless, switching methods from HPLC to UHPLC equipment and vice versa is a powerful tool to increase laboratory efficiency. Modern systems with two flow lines in one system aim to simplify the transition between the two techniques.
Photo Credit: Gines Valera Marin/Shutterstock.com

Gesa Schad, Shimadzu, Duisburg, Germany
Although ultrahigh-pressure liquid chromatography (UHPLC) method development is fast and cost efficient, many of the analytical methods used in quality control laboratories are still conventional high performance LC (HPLC) separations. Transferring these HPLC methods to UHPLC and validating them is a time-consuming and labour-intensive task. Nevertheless, switching methods from HPLC to UHPLC equipment and vice versa is a powerful tool to increase laboratory efficiency. Modern systems with two flow lines in one system aim to simplify the transition between the two techniques.
In recent years, there has been a strong focus on the use of ultrahigh-pressure liquid chromatography (UHPLC) instruments and small particle columns to develop faster and better analytical methods to improve efficiency and throughput, especially in R&D environments. Yet many of the analytical methods used in quality control laboratories, including those listed in the Pharmacopoeia, are conventional high performance LC (HPLC) methods. Transferring these HPLC methods to UHPLC and validating the new methods is a time-consuming and labourâintensive task, which is why many companies don’t do it.
However, new method development has to be faster and more cost efficient, so UHPLC equipment is often used. After high-throughput screening, identification, and optimization of the final method, the separation conditions are transferred back to normal HPLC mode to be run in the QA/QC departments that still prefer the robust and reliable HPLC equipment. This method transfer poses some problems because of differences in system volume and therefore gradient delay. The gradient delay volume causes an isocratic hold before the beginning of the gradient, which affects the separation selectivity. It can differ from system to system and therefore complicates the transfer of a gradient method from one system to another.
Straightforward Method Transfer
With the rise of UHPLC and the objective of faster, more efficient separation methods, chromatographers face the challenge of transferring routine HPLC methods to UHPLC equipment, or methods developed on a UHPLC screening system to HPLC instruments for routine use. Just like any other task, method transfer to a different instrument and different column dimensions is fairly straightforward when done in accordance with a few simple rules. UHPLC equipment has to withstand higher pressures and the system volume is optimized for minimum gradient delay and extracolumn band broadening.
HPLC instruments are designed to work at a pressure no higher than 400 bar, so column dimensions of 150 or 250 × 4.6 mm with a flow rate of 1 mL/min and particle sizes of 5- or 3-µm are used. These conditions allow for peek tubing with an inner diameter of 0.3 mm (blue) because system and gradient delay volume are not crucial factors with high flow rates and long gradient times (see Figure 1).
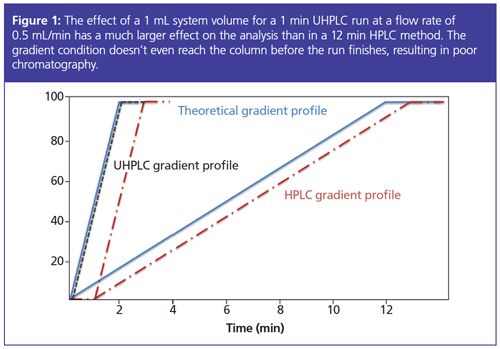
Hybrid Options
Hybrid systems are available that incorporate two analytical flow lines with different system volumes in a single instrument. By switching between these flowlines, both HPLC and UHPLC analyses can be performed with the same system. The relative separation pattern is preserved by software that adjusts the gradient conditions according to changes in flow rate and column dimensions, and enable automated compensation for differences in system volume.
This technology helps less experienced users in any method transfer efforts, and also allows existing HPLC or UHPLC methods run on any other instrument platform to be matched, eliminating the need to consider and carefully adjust tubing lengths to achieve identical system volumes between instruments.
Experimental
Method Conditions: Column: 150 × 4.6 mm ACE Excel 3 SuperC18 (ACT); mobile phase: A: 0.1% formic acid in H2O; B: 0.1% formic acid in MeCN; gradient programme: time: 0 min, % B: 20; time: 20 min, % B: 70; time: 25 min, % B: 70. This was followed by 11 min reâequilibration time (20 %B) 36 min run time; flow rate: 1 mL/min; temperature: 40 °C; detection at: 254 nm; sample: 0.6 mg aspirin, 0.1 mg sulindac and 0.2 mg of naproxen, flurbiprofen, phenylbutazone in 1 mL acetonitrile–water (50:50 v/v); system: Nexera-i MT method transfer system with photo diode array detector (Shimadzu).
Method Transfer
When transferring an HPLC method to a UHPLC system or vice versa, care must be taken to maintain selectivity when changing column dimensions and flow rate. In a gradient separation the crucial factor is the relative retention factor (k*) that is influenced by gradient time (tg), flow rate (F), change in organic solvent (â%B), column dead volume (V0), and a constant (S) according to:
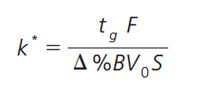
[1]
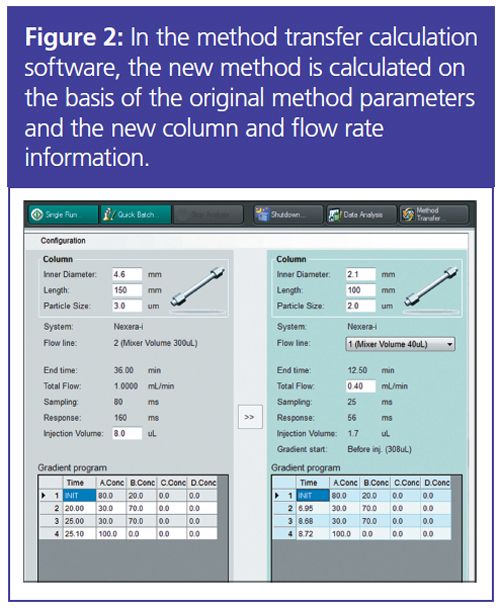
The relative retention factor (k*) must be kept constant, and so gradient time has to be adjusted when column dimensions or flow rate are changed. However, other parameters, such as injection volume or detector capture rate, also need to be adjusted when looking to use a smaller column or increase the speed of the analysis. The new method is calculated based on original method parameters and the new column and flow rate information (Figure 2).
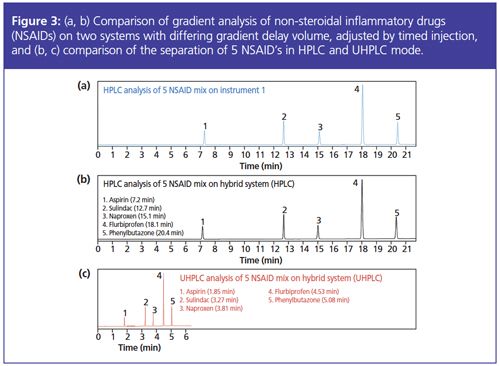
The example shows the successful transfer from a 36-min HPLC method to a 7-min separation in UHPLC conditions, where gradient time, column dimensions, and flow rate were adjusted to maintain selectivity in the faster analysis (Figure 3 [b–c]). It doesn’t take an expert chromatographer to perform this kind of increase in efficiency.
Conclusion
The transfer of HPLC methods to UHPLC equipment and vice versa is a powerful tool to increase laboratory efficiency or to allow high speed method development of methods that have to be run on HPLC equipment. However, when changing from one to the other, care must be taken to consider the differences in the two techniques. Systems with two flow lines in one system can simplify the method transition process and avoid problems associated with gradient delay.
Gesa Johanna Schad graduated with a Diploma in chemical engineering from the Technical University NTA in Isny, Germany, in 2004 and as a Master of Science in pharmaceutical analysis from the University of Strathclyde in Glasgow, UK, in 2005. Worked until 2006 as a consultant in HPLC method development and validation in an analytical laboratory of the FAO/IAEA in Vienna, Austria. She gained her doctorate for research in pharmaceutical sciences at the University of Strathclyde in 2010 and was employed as HPLC specialist in the R&D department at Hichrom Ltd. in Reading, UK from 2009. Since 2013, she worked as a HPLC product specialist and since 2015 as HPLC Product Manager in the analytical business unit of Shimadzu Europa in Duisburg, Germany.
E-mail:shimadzu@shimadzu.euWebsite:www.shimadzu.eu

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








