The Fundamental Shift to Tandem Mass Spectrometry
Special Issues
In this article, we examine how tandem and tandem hybrid mass spectrometry has opened up new frontiers already. We go further and examine how lesser-known experiments are breaking new ground, with alternative fragmentation techniques, as well as the addition of extra levels of orthogonality by parallel separations techniques.
In this article, we examine how tandem and tandem hybrid mass spectrometry has opened up new frontiers already. We go further and examine how lesser-known experiments are breaking new ground, with alternative fragmentation techniques, as well as the addition of extra levels of orthogonality by parallel separations techniques.
Today, in the biopharmaceutical industry mass spectrometry (MS) is a critically useful and efficient tool for routine and investigational analysis in therapeutic discovery, development, and production. Almost every analytical department now routinely uses MS at some stage in the process of therapeutic development.
However, there is one intriguing aspect that is somewhat surprising given the prevalence of MS; that is, a monolithic view held by some whereby all mass spectrometers or all techniques are lumped into a broad category labeled "MS." This is all the more surprising given that the experiments performed are enormously varied. It is the view of these authors that such convenient shorthand results from a predominance of a small number of MS experiment types adopted by the industry. Although many may know that alternative experiments exist, few have the time to explore them and many may be unaware of the extreme utility of these experiments for greater efficiency and information, with little time penalty or method development. In this article, we touch on how tandem mass spectrometry (MS-MS) has developed and how alternative uses of it may better inform the industry and speed up therapeutic design and development, with particular reference to the biopharmaceutical area.
A Brief View of History
The development of MS-MS has not been seen as obvious, and has relied partly on fortuitous results and typical scientific curiosity about fundamental gas phase reactions (1,2). In the 1970s the use of MS-MS was extremely informative about the behavior of ions in the gas phase and their dissociation, although it remained highly academic (3,4). In experiments that often used enormous magnetic sector instruments, advanced research was still looking intently at what was later termed "fundamentals," reflecting how the field was aiming to understand the very mechanisms of what was occurring (5). In fact, MS-MS research had been a steady thread of activity right from the very start of mass spectrometry, beginning more than a century ago (1). However, the 1970s saw the massive rise of a plethora of instrument types, including some ambitious multiple-sector instrumentation. One type was the tandem quadrupole, which opened up what has arguably been the most commercially successful type of mass spectrometer ever invented, and which still dominates the market today (8). In common terminology, this has become known as a triple quadrupole, on the basis that the middle quadrupole segment was the collision cell, although this mechanism has long been superseded.
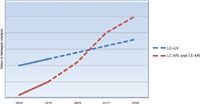
Figure 1: Graph showing the estimated relative adoption rates of MS-based detection versus optical detection for the biopharmaceutical market (2014â2018). Both techniques grow above 5% per annum, but MS-based techniques accelerate as more biotherapeutics reach the market and pipeline. (Data sources: various, including FiercePharma, PhRMA reports, public company reports; collated by the author.)
But here too lies one of the continuing puzzles for many people in the field: Why has the variety of experiment types not been used more widely? The most predominant MS-MS experiment remains that used for quantification of analytes: multiple reaction monitoring (MRM), whereby a precursor is selected, and a small subset of the fragments are subsequently monitored to determine very precisely how much of the analyte is present — mostly with reference to isotopically labeled standard analog species. However, almost all tandem mass spectrometers have the inherent capability of looking "backward" by using the fragment ion species to reconstruct what the precursor molecule was like. This has been extensively explored in the metabolite identification world — for example, where predictable biotransformations can be mapped by integrating the MS and MS-MS information with informatics packages (10). Additionally, it is also possible to look backward and mark out the parts of a molecule that are not present because they didn't ionize or were broken into pieces that are not recognizable. Examples of this are "constant neutral loss" experiments, or "parent–precursor ion scans" (13). It is all the more surprising that these types of experiments are not performed more frequently because they can be done almost simultaneously in certain types of mass spectrometers (for example, tandem quadrupoles and quadrupole time-of-flight [QTOF] systems).
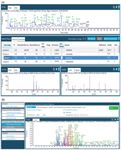
Figure 2: (a) An example of an automatically assigned peptide map by LCâMS-MS where each of the peaks is labeled by software. The panels at the bottom indicate the orthogonal evidence available to the reviewer in the event of queries. The coverage achieved was 98% over the 60-min run. (b) An example of an automatically assigned peptide map of the molecule trastuzumab by capillary electrophoresis electrospray ionization (CESI) separation with a color-coded assignment of the peptides identified (100% coverage).
Tandem in Space and Time
Here too, the subtle differences in what tandem mass spectrometers actually are is instructive: tandem itself is a conflation of "tandem in space" and "tandem in time." The examples all mentioned above are of the tandem-in-space type, whereby the instrument components that analyze each of the successive fragments are separated physically. This is not the case for instruments that are "trapping," in which ions can be stored for some period of time, potentially forever. This distinction is important, because the differences in tandem types have largely determined which types of instruments have been sold for specific applications in the pharmaceutical market. Traps that store specific ions and subject them to even more analysis are extremely good at homing in on one specific thing, and providing an extremely in-depth view of what that analyte is — even though it may be at the expense of other molecules present simultaneously. On the other hand, tandem-in-space instruments are extremely well suited for looking at a number of things simultaneously, perhaps at the expense of losing detail on specific items. So, why not have a combination of both attributes? In many ways, this is partly where the instrumentation industry has headed, with some surprisingly creative results.

Figure 3: Graphical illustration of data acquisition using informatics software (Swath, AB Sciex) where precursor and fragment ions are collected through an entire experiment. This technique provides a comprehensive dataset in a peptide map that can be remined at a later date with new hypotheses or to extract information that was not predicted. For more information see reference 16.
Economically Viable Adoption
By the time the tandem quadrupole was being widely adopted for clinical work in the mid 2000s, it was already profiting from rapid advances, so the invention of what many have described as a new "class" of instrument was also well accepted (9). But additionally, by this stage many researchers were working directly with the nascent biopharmaceutical industry and were extremely oriented toward the actual samples that needed to be analyzed. In an example of this, Hopfgartner and colleagues (9) commented:
". . . the uniqueness of the instrument is that the same mass analyzer Q3 can be run in two different modes [quantitative and qualitative] . . . [one mode,] EMC . . . offers obvious advantages, in particular for samples containing very low peptide levels. For many analytical challenges, selectivity often becomes more important than sensitivity."
In that article, Hopfgartner and colleagues also compared the capabilities of trapping with "axial" ejection, which coped with the limited storage capacity of three-dimensional (3D) traps. The limited storage capacity can be a hindrance when a mixture of large, highly charged species are examined together, where the ions that are more predisposed to become highly charged may force the exclusion of lower abundance species from the spectrum. In instruments with "linear" ion traps, it is one of the reasons why low relative abundance is not as problematic (9,13). The capability to sequence peptides of low abundance was noted.
Perhaps two of the answers as to why more MS-MS experiment types are not used are profit reasons and the relative slowness in the rise of truly intuitive software. The profit aspect relates to economic imperatives that determine whether instruments are able to provide value for money — and of course in constrained economic times this is all the more apparent. These authors suggest that as we move forward, greater emphasis will be placed on the biopharmaceutical industry obtaining tools that are able to simultaneously provide extensive experiment types — productivity — as well as allow post-experiment investigation — security. Now that many experiment types are instantaneous within the constraints of typical experimentation, the ability to go back to find data that may have always been there becomes much more feasible. In fact, many of these advances have been quietly adopted as standard tools, including the ability to automatically assign peptide sequences and look at the sequence simultaneously (14). The use of informatics has progressed, but perhaps by virtue of not being as emotionally exciting as advances in expensive capital equipment, it is paradoxically perceived as less exciting. But there has been a crucial development in the industry: the ability to go back and re-examine data with fresh hypotheses without new sample injections. So, the combination of instrument capability and informatics has provided some real benefits, not least of which is the automation of many tedious tasks otherwise performed by humans.
Conclusion
The practical application of MS-MS has long been recognized, and a subset of the experiments has long been a default tool in the industry (MRMs). But it is reasonable to argue that the depth of capability has remained untouched. It can be argued that it is economic imperatives that push organizations to start to use more of the tools already at their disposal to obtain more out of their capital expenditures. In some cases, this might mean finding contaminant host cell proteins more rapidly, or identifying previously unknown sequence variants in an effort to improve product quality as quickly as possible (15). Under all circumstances, MS-MS will only increase in value, usage, and, understanding.
St. John Skilton, PhD, is a senior global marketing manager for biologics with AB Sciex in Framingham, Massachusetts. Eric Johansen, PhD, is a global technical marketing manager for biopharmaceutical applications at AB Sciex. Xu Guo is an application scientist in the product application lab at AB Sciex. Direct correspondence to: stjohn.skilton@absciex.com
References
(1) http://www.asms.org/docs/history-posters/tandem-ms-poster-2012.pdf?sfvrsn=2.
(2) R.G. Cooks and J.H. Beynon, J. Chem. Educ. 51(7), 437–43 (1974).
(3) E. Gustafsson and E. Lindholm, Arkiv foer Fysik 18, 219–39 (1960).
(4) J.H. Beynon et al., Int. J. Mass Spectrom. Ion Phys. 3(5), 313–21 (1969).
(5) F.W. McLafferty, D.J. McAdoo, and J.S. Smith, J. Am. Chem. Soc. 91(19), 5400–1 (1969).
(6) A.L. Yergey, J.R. Coorssen, P.S. Backlund, P.S. Blank, G.A. Humphrey, J. Zimmerberg, J.M. Campbell, and M.L. Vestal, J. Am. Chem. Soc. 13(7), 784–791 (2002).
(7) B.A. Mamyrin, V.I. Karataev, D.V. Shmikk, and V.A. Zagulin, Zh. Eksp. Teor. Fiz. 64(1), 82–9 (1973).
(8) M.L. Vestal and J.H. Futrell, Chem. Phys. Lett. 28(4), 559–61 (1974).
(9) G. Hopfgartner, E. Varesio, V. Tschäppät, C. Grivet, E. Bourgogne, and L.A. Leuthold, J. Mass Spectrom. 39, 845–855 (2004). DOI: 10.1002/jms.659. Available at: http://medchem.rutgers.edu/AnalMedChem511/pdf_files/RB_pdf/LIT%20and%20QQQ.pdf.
(10) G. Hopfgartner, I.V. Chernushevich, T. Covey, J.B. Plomley, and R. Bonner, J. Am. Soc. Mass Spectrom. 10, 1305 (1999).
(11) G. Hopfgartner and F. Vilbois, Analusis 28, 906 (2001).
(12) J.W. Hager, Rapid Commun. Mass Spectrom. 17, 1389 (2003).
(13) I.V. Chernushevich, A.V. Loboda, and B.A. Thomson, J. Mass Spectrom. 36, 849–865 (2001).
(14) L.C. Gillet, P. Navarro, S. Tate, H. Röst, N. Selevsek, L. Reiter, R. Bonner, and R. Aebersold, Mol. Cell. Proteomics 11(6), PMID 22261725 (2012).
(15) J. Hogan and S.J. Skilton, "Efficiency Gains with Sequence Variant Analysis by Mass Spectrometry," Genet. Eng. Biotechnol. News webinar (2014). Available at: http://www.genengnews.com/webinars/efficiency-gains-with-sequence-variant-analysis-by-mass-spectrometry/219/.
(16) http://www.absciex.com/applications/biomarker-discovery-and-omics-research/swath-acquisition.
(17) http://www.absciex.com/Documents/Applications/HCP_Tech_Note%20_Detailed_FINAL.pdf.

Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Quantifying Microplastics in Meconium Samples Using Pyrolysis–GC-MS
March 26th 2025Using pyrolysis-gas chromatography and mass spectrometry, scientists from Fudan University and the Putuo District Center for Disease Control and Prevention detected and quantified microplastics in newborn stool samples.










