FPLC versus Analytical HPLC: Two Methods, One Origin, Many Differences
Fast protein liquid chromatography (FPLC) and high performance liquid chromatography (HPLC): two liquid chromatographic methods in direct comparison. This article discusses the differences between the two techniques with particular emphasis on the requirements of their respective analytes.
Photo Credit: olandesina/Getty Images
Stephanie Runde, Knauer Wissenschaftliche Geräte GmbH, Berlin, Germany.
Fast protein liquid chromatography (FPLC) and high performance liquid chromatography (HPLC): two liquid chromatographic methods in direct comparison. This article discusses the differences between the two techniques with particular emphasis on the requirements of their respective analytes.
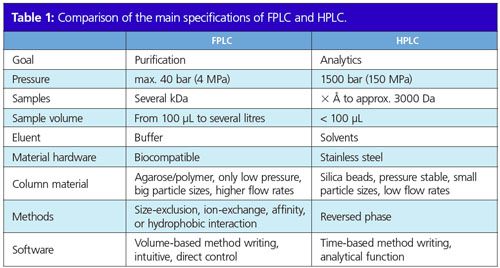
The names fast protein liquid chromatography (FPLC) and high performance liquid chromatography (HPLC) offer a hint to the differences between these chromatographic methods. While HPLC works with high pressure to analyze small chemical compounds, FPLC purifies large biomolecules like proteins or DNA. The chromatography of biomolecules is very demanding and sensitive because they cannot stand high temperatures, high pressures, or the solvents usually used in HPLC. For these reasons, the separation of biomolecules requires an alternative approach to HPLC.
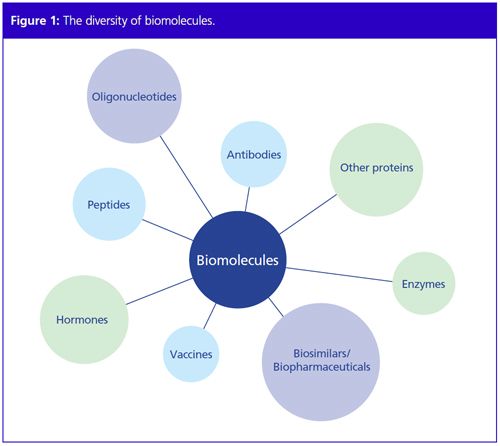
Several terms are commonly used for fast protein liquid chromatography such as biochromatography, bioseparation, or biopurification. This special form of chromatography is applied to purify large biomolecules of several kilodaltons (kDa) such as proteins, nucleotides, or peptides (Figure 1). The aim of the FPLC user is to obtain as much pure and native product as possible. The aims of classical HPLC, on the other hand, are to identify and qualify analytes, usually small compounds ranging in size from a few atoms up to approximately 3000 Da.
The Challenge of Getting a Pure Protein from a Cell Extract
Typically, biomolecules are purified from bacterial or eukaryotic cells, which are packed with proteins, DNA, RNA, and cellular membranes. Hence, the purification of the protein of interest out of a cell extract can be very challenging. Biochemists apply several tricks: one is to use recombinant proteins, which are overexpressed by the cells. Overexpression of the protein of interest enables purification in larger quantities. The second trick is to add a tag to the protein of interest, which is specifically recognized by the resin (column material). Proteins without a tag will not bind to the column and elute immediately, while the desired protein is enriched and can be separated easily.
Biomolecules are purified from cellular lysates, which means much larger sample volumes than in analytical HPLC. Therefore, bigger sample loops or even pumps with higher flow rates are used to inject the sample. Moreover, FPLC and HPLC column materials differ completely. For HPLC, silica beads with very small particle sizes and with a large resistance to high pressures are applied, while FPLC requires agarose or polymer material with bigger particle sizes for most methods. The resins used for FPLC are not as pressure stable as silica beads and are very sensitive to air bubbles. Furthermore, not only the column material but also the column hardware differs. In classical HPLC, pressure-resistant stainless steel columns are used. As already mentioned, pressure stability is not important for FPLC and so it is possible to work with transparent and biocompatible glass columns. This is a great advantage since the user can check the column for air bubbles or monitor the condition of the material during a purification run.
Diverse Biomolecules, Diverse Purification Methods
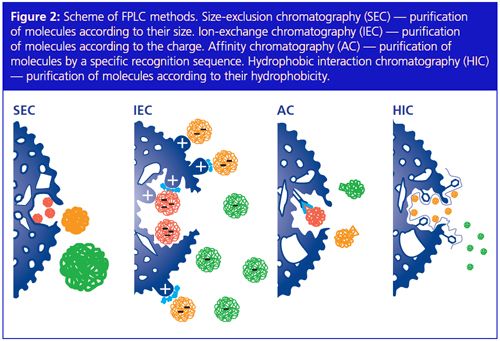
The differences in the column material also reflect in the methods. In analytical HPLC, reversed phase chromatography with hydrophobic stationary phases and polar mobile phases is the method of choice, while in FPLC a greater variety of methods are applied (Figure 2). One FPLC method is size-exclusion chromatography (SEC) where molecules are separated according to their size. Smaller molecules can diffuse into the pores of the beads, while larger molecules pass through the column almost unretained. The smaller molecules elute later from the column generating a gradient of these molecules according to their size. Another separation approach is ion-exchange chromatography. Biomolecules are separated and purified according to their specific charge, which depends on the pH of the buffer. The more charged the protein is, the better it will bind to the opposite charged resin. To elute the protein from the column the concentration of salt ions is increased during the run. The salt ions compete with the protein for binding to the resin. Another important FPLC method is affinity chromatography where the molecule of interest can bind specifically to the column while the other molecules cannot and will elute without binding. Here columns with special media that recognize the desired biomolecule are used. A special form of affinity chromatography is immobilized metal ion affinity (IMAC). The protein of interest has to be genetically altered by adding a tag to the protein, which usually consists of six histidines. The IMAC resin specifically recognizes this “Hisâtag”. Elution takes place by increasing the concentration of imidazole that competes with the His-tagged proteins. Moreover, proteins can also be separated by their specific hydrophobicity using the hydrophobic interaction separation method. The resin is composed in such a way that the most hydrophobic proteins will bind the strongest to the column and are eluted by decreasing the salt gradient.
In order to purify a desired protein, a combination of methods is usually used. In the first step, the so-called “capture” step, the protein is purified from the crude extract. Typically, affinity chromatography is used for this first step of the purification. The second step, the “intermediate” step, removes further contamination by ion-exchange chromatography or hydrophobic interaction. The aim of the final “polishing” step - usually a sizeâexclusion chromatography step - is to get rid of all remaining impurities to gain a high purity product. This protein purification strategy depends totally on the specific biomolecule. In some cases, a two-step purification can be sufficient. The more methods are combined, the more of the protein of interest is lost during the purification, but a higher purity can be achieved.
After the purification, the attained sample can be analyzed for purity, concentration, and enzyme function or activity using HPLC, sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), enzyme-activity assays, or mass spectrometry.
FPLC Requirements
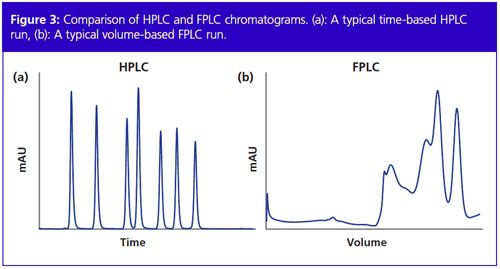
Proteins are composed of amino acids lined up as a chain. This chain is folded into a three-dimensional structure, which is the key to the function and activity of each protein. Therefore, it is very important to maintain this protein structure during the purification process. External factors like high temperature, high pressure, extreme pH, or solvents can disturb the protein structure and are therefore avoided in FPLC. The working temperature for proteins is usually 4 °C. This is why an FPLC machine is often placed inside a cold chamber or cold room where it is exposed not only to low temperature but also to condensing humidity. The components of an FPLC system have to be specially designed for these conditions. Moreover, FPLC components encounter an additional challenge, namely saline buffer solutions that are used as eluents. Isosmotic pH and salt concentrations similar to the cell environment are usually chosen. Chromatography systems are generally made of stainless steel. On the one hand, the salt in the buffers can lead to corrosion and on the other hand the metal ions from the stainless steel can interfere with the protein and disturb its structure. Therefore, it is important to avoid stainless steel and use biocompatible material like polyether ether ketone (PEEK) ceramic or titanium when performing FPLC. Like HPLC systems, FPLC systems are also controlled by software. There are considerable differences between FPLC and HPLC software. The latter is mainly used to analyze the samples and contains a lot of analytical tools. In FPLC however, not many analytical tools are needed and it is common to generate methods based on volume or even column volume (Figure 3). For most FPLC applications, column volume-based methods are preferred, making upscaling easy. FPLC software is mostly very intuitive and user-friendly and includes direct control, which allows adjustments of the parameters during a run. Thereby, the user can react upon different situations very spontaneously.
It is therefore clear that there are major differences between these two chromatographic areas (Table 1). Methods, hardware, and software differ greatly depending on whether the molecule is analyzed or purified. For FPLC applications the sensitive nature of the samples and the ambition to keep them as native as possible adds to the challenge. Overall, both techniques are highly interesting areas that everybody working in the field should be aware of.
Stephanie Runde graduated from the Technical University of Munich, Germany, with a diploma in biochemistry. She obtained her PhD at Freie Universität Berlin, Germany, and currently works at Knauer Wissenschaftliche Geräte GmbH as product manager for FPLC.

Determining the Effects of ‘Quantitative Marinating’ on Crayfish Meat with HS-GC-IMS
April 30th 2025A novel method called quantitative marinating (QM) was developed to reduce industrial waste during the processing of crayfish meat, with the taste, flavor, and aroma of crayfish meat processed by various techniques investigated. Headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) was used to determine volatile compounds of meat examined.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.
Using GC-MS to Measure Improvement Efforts to TNT-Contaminated Soil
April 29th 2025Researchers developing a plant microbial consortium that can repair in-situ high concentration TNT (1434 mg/kg) contaminated soil, as well as overcome the limitations of previous studies that only focused on simulated pollution, used untargeted metabolone gas chromatography-mass spectrometry (GC-MS) to measure their success.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)










