Fast Gas Chromatography
LCGC Asia Pacific
Fast gas chromatography (GC) has received new attention recently in the form of available enhanced instrument capabilities. This instalment reviews important characteristics and requirements of fast GC: What can fast GC do for separations, and how can laboratories take advantage of enhanced separation speeds?
John V. Hinshaw, GC Connections Editor
Fast gas chromatography (GC) has received new attention recently in the form of available enhanced instrument capabilities. This instalment reviews important characteristics and requirements of fast GC: What can fast GC do for separations, and how can laboratories take advantage of enhanced separation speeds?
Fast gas chromatography (GC) has received renewed attention in the past two years. New GC instruments and accessories in 2015–2017 include more capabilities for fast GC than their predecessors, such as rapid oven heating and cooling, extended inlet pressures, support for hydrogen carrier gas, and faster detector response times. Beyond these basics, workflow features such as ease of changing columns, energy efficiency, reduced maintenance, and improved user interfaces have also garnered significant attention. As older instruments age out and replacements are installed, laboratories may access new abilities to obtain faster separations and sample throughput. On the surface, these changes to the GC laboratory landscape resemble the long-coming transition from packed to capillary columns of the 1980s and 1990s. However, this time users may have the choice to run their separations faster with their new instrumentation, or to keep going as before and leave their capillary column methods unchanged. No doubt most will opt for the second scenario at first, but, as with a new automobile, sooner or later operators will explore how fast the new equipment can perform.
What Is Fast GC?
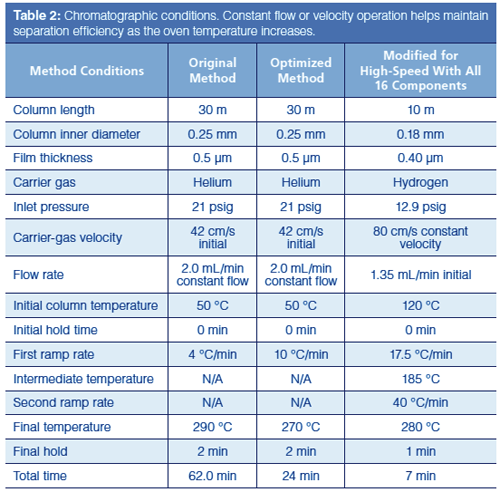
As applied to GC, “fast” and “highâspeed” are not well defined. These terms are relative and are meaningful only when compared to an existing state. “Optimized” is a related comparator that implies a limited change. For the purposes of this article, optimized separations would be achieved without modification of the column dimensions or instrumentation, whereas fast or high-speed separations imply selection of specific column dimensions and instrument options that can produce significantly faster separations. So for example, if an existing separation’s last peak of interest is eluted in 25 min, it might be optimized by increasing the carrier flow and temperature ramp rate, thereby causing the run time to decrease to 15 min. The same separation might be upgraded to high speed by reducing the column inner diameter, changing to hydrogen carrier gas, and ramping the column oven at higher speeds. Achieving this higher speed might require a high-power oven heating arrangement or an add-on high ramp-rate module, plus faster detector response times and injection speeds to match.
Pushing a separation to higher speeds has both positive and negative impacts. Reducing the total run-cycle time-the interval from one sequential injection to the next-directly increases sample throughput, which can be a significant advantage in laboratories with high sample loads. But changing the separation method to run at higher speeds can be a daunting and time consuming effort. Careful method optimization followed by required validation is still necessary. Having newer instrumentation already in the laboratory that is capable of high speed removes some of the expense, and this barrier will become lower over time. Deciding whether to optimize an existing separation or to proceed further into high-speed operation is fast becoming more of a business decision than a choice to implement a new and untried technology in the lab.
How to Achieve Faster Separations
After the decision is made to migrate a separation to higher speeds, the process of getting there is fairly well defined, although there are some pitfalls on the way. The first step is to pick a goal for how much faster the separation could be to realize significant gains. For an example, we will use the synthetic chromatogram of pesticides in Figure 1. Chromatogram modelling software makes it easy to test out scenarios for optimizing and speeding up complex mixture separations; in this case I used the Pro EZGC Chromatogram Modeler (Restek Corporation) (1).
GC simulation software comes in two flavours. First there is flow calculator software that uses the same calculations as builtâin electronic pressure control to compute column flows, pressures, and velocities. Several programs are available, and most include a simple method translation mode that attempts to predict or maintain peak elution order as changes to columns and conditions are made. The second type of simulation software includes the flow calculations but goes beyond that by accessing a database of compound retention behaviour on specific stationary phases. Typically, the data are obtained by measuring two or three separations under different thermal conditions for each compound on each stationary phase in the database. These thermodynamic data are then used to estimate each peak’s retention behaviour under a wider range of conditions and column dimensions. Consideration of the influence of flow and column dimensions on peak widths is mixed in with retention information to produce an estimate of peak resolution for each peak pair.
As we will see, going from just optimizing the separation to highâspeed operation using a narrower-bore column may be quite beneficial. The chosen test compounds listed in Table 1 correspond to a pesticide sample that I separated in the 1990s as part of a temperature program optimization exercise, work that was subsequently described in an early “GC Troubleshooting” instalment (2). For those readers who are familiar with this type of sample, bear in mind that the conditions we will arrive at won’t necessarily resolve the entire set of analytes. This is only an illustration: As always, carefully check and validate methods as they are optimized or converted to the high-speed realm.
Initially, the optimization exercise focused on the last 10 peaks of the original sample. Then, for conversion to a column with a smaller internal diameter, I included all 16 of the compounds from my original experiments, changed to a smallerâinner-diameter column with hydrogen carrier gas, and ran the simulation program’s temperature refinement option. See the end of the instalment for the results.
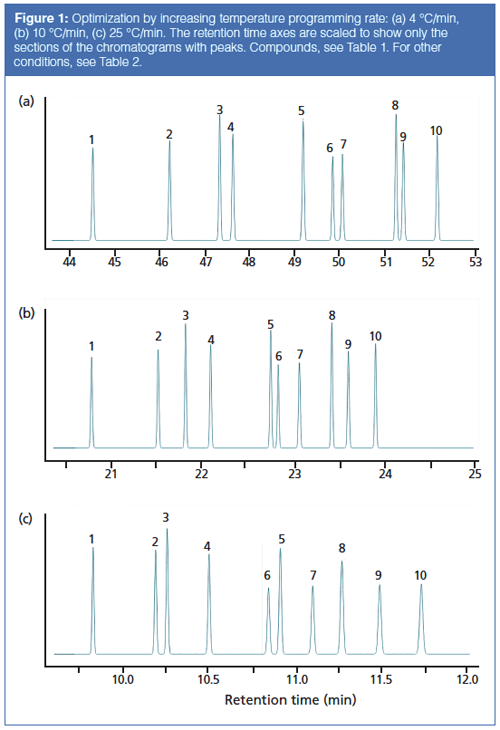
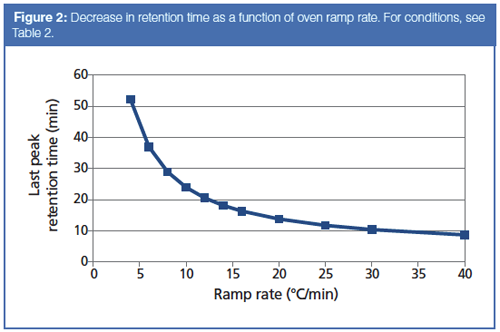
The starting and optimized GC conditions are given in Table 2, and the corresponding chromatograms are shown in Figure 1. The original chromatogram was obtained at the slowest simulated ramp rate of 4 °C/min. The 10 selected peaks are well resolved in a total run time of 62 min. Increasing the ramp rate stepwise from 4 °C/min to 40 °C/min causes the retention time of the last peak to decrease from 52.2 min to 8.7 min, as illustrated in Figure 2. At first glance this is a very significant speed-up, but it is not all that it seems to be.
First of all, peaks 5 and 6 merge together as the ramp rate increases through 14 °C/min. At higher ramp rates they are separated again but their elution order reverses. This well known effect stems from chemical differences between the peaks that produce differential retention effects as temperatures shift. It is often observed on moderately polar columns such as the 35% phenylmethylsilicone phase used here. Peaks 3 and 8 exhibit similar behaviour as they migrate between the surrounding peaks with increasing ramp rates.
The resolution of peaks 2 and 3 reaches a maximum at 12 °C/min and then decreases as the ramp rate heads out towards 40 °C/min. Figure 3 shows the predicted resolution of two peak pairs across the entire range of programming rates. An important goal of an optimization exercise is to maintain a minimum resolution of 1.5, which is indicated by the horizontal red line in Figure 3. Imposing this limitation, ramp rates between 12 °C/min and 20 °C/min have to be excluded.
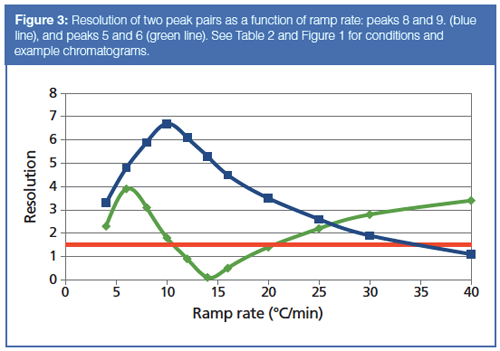
A second problem arises when the ramp rate reaches 25 °C/min or higher: All of the peaks are eluted within a few degrees of the final oven temperature. Illustrated in Figure 1(c), this situation indicates that the oven ramp rate is too fast for the carrierâgas flow rate. It has gotten ahead of the peaks’ progress through the column, and the peaks no longer interact as much as they could with the stationary phase. The highest oven temperature is determined by the column’s upper programming limit, which in this case is 290 °C. Effectively, the optimal programming rate for this separation is 10 °C/min, as shown in Figure 1(b). Elution at the final oven temperature plus loss of resolution make higher ramp rates less desirable. Thus, we can realize about a factor of two increase in separation speed for this sample without changing the column or modifying the carrier-gas flow.
A different set of conditions or different column dimensions should be considered when most of the peaks are bunched at the maximum temperature. In this case, increasing the carrier-gas flow or velocity would move the selected peaks to lower retention times. At the same time, the programming rates at which resolution for all peaks exceeds 1.5 will shift. The ramp-rate optimization exercise can be repeated at higher flows to find potential new values and perhaps get to even higher speeds, possibly further improving the separation of this 10-component subset of the original pesticide sample.
Even Faster
I decided instead to jump to a higher-speed column to see just how fast the separation might be performed. The result is shown in Figure 4, where a 10 m × 0.18 mm column with a 0.2-µm film of the same stationary phase accomplishes the separation of the 10 selected compounds in under 2 min! The ramp rate was increased to 70 °C/min and the initial carrier velocity to 80 cm/s. With a GC system capable of running this faster method and column, a speed increase of about 25 times might be achieved. Significantly, the initial temperature was increased to 150 °C, which also saved some time. This last change, however, is not compatible with separating the entire 16-component mixture because the first six peaks would be crammed together at the start and poorly resolved, if at all.
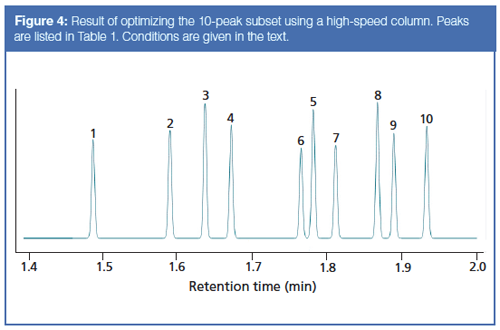
In addition, this type of sample usually occurs at trace levels, and splitless or on-column injection may be required. That in turn needs a lower initial oven temperature to realize a solvent effect and solute trapping of the earlier peaks, so the higher initial temperature is not very realistic. Still, the achievable speed is impressive.
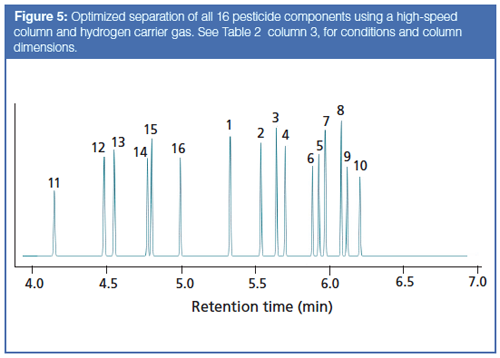
For a final try, I included all 16 compounds and optimized for speed with the same narrow-bore column except for a thicker film of 0.4 µm, and a lower initial temperature, a necessary change to resolve all 16 peaks. Figure 5 illustrates the result. The run time needed is about 6.25 min to resolve all of the peaks. A two-ramp temperature program was used to split the separation into a first section for the additional six peaks, and a second section at a higher ramp rate to resolve the 10 peaks of interest from before.
Conclusion
At this point, going any further would require a trip to the laboratory to measure how real separations stack up to the predictions of the GC simulations. I can say that the simulated results given here match fairly well with the measured chromatograms from 1991, although those were performed with different column inner diameter, length, and film thickness. At the time, I found that the critical peak pair 5 and 6 were coeluted at between 8 and 10 °C/min, and peaks 2 and 3 also showed behaviour similar to the simulation. Given the observed peak reversals and the range of conditions in which peak resolution can drop below 1.5, no conclusions about optimization or conversion to highâspeed separation can be taken away from the kind of exercises presented here. Such a journey through simulation is very informative, but there is no substitute for injecting and recording real chromatograms.
References
- Restek Corporation (State College, PA, USA), Pro EZGC Chromatogram Modeler, http://www.restek.com/proezgc, October, 2017.
- J.V. Hinshaw, LCGC9(7), 470–473 (1991).
“GC Connections” editor John V. Hinshaw is a Senior Scientist at Serveron Corporation in Beaverton, Oregon, USA, and a member of LCGC Europe’s editorial advisory board. Direct correspondence about this column to the author via e-mail: LCGCedit@ubm.com

Determining Enhanced Sensitivity to Odors due to Anxiety-Associated Chemosignals with GC
May 8th 2025Based on their hypothesis that smelling anxiety chemosignals can, like visual anxiety induction, lead to an increase in odor sensitivity, a joint study between the University of Erlangen-Nuremberg (Erlangen, Germany) and the Fraunhofer Institute for Process Engineering and Packaging (Freising, Germany) combined behavioral experiments, odor profile analysis by a trained panel, and instrumental analysis of odorants (gas chromatography-olfactometry) and volatiles (gas chromatography-mass spectrometry).
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.
Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)





