Early Petroleum Chromatographers
LCGC North America
This column deals with a few chemists active during the early growth of the petroleum industry who carried out chromatography-like investigations even before Tswett...
Evolution in science and technology is usually gradual and major inventions and achievements have their forerunners — observations or techniques that seem to be close to the concept of the later invention. This is also true about chromatography. Today, it is without any dispute that Mikhail Tswett was the true inventor of this method (1). However, before his groundbreaking activities from 1903 to 1906, there were others who, intentionally or unintentionally, carried out some separation in a moving flow. An example is Runge, who, about one-half a century before Tswett and a century before the invention of paper chromatography by Martin's group, presented dynamic separation on filter paper, although not for the purpose of separation but to illustrate reactions (2).

Classical chromatography has been based upon adsorption, and adsorption processes have been used in laboratory as well as in manufacturing before the introduction of chromatography. Some of these processes were carried out in a moving stream and their aim was usually the removal of some undesired compounds, in other words, some kind of fractionation or separation. Such processes also have been introduced in petroleum processing: one example is the production of Vaseline from crude oil. However, we do not conceive these manipulations as chromatography.
Toward the end of the 19th century, the rapidly evolving petroleum production also initiated speculations concerning the origin of petroleum, and attempts have been made to explain the reason for the obvious difference in the characteristics of crude oils occurring at different locations. One prominent American chemist involved in such work was David T. Day, whose theory (the "filtration hypothesis") was based upon the assumption of migration of the oil in the earth through various strata and selective retardation of certain compounds or compound groups during this migration. To prove the validity of his assumption, Day carried out some model experiments and demonstrated that indeed, some fractionation can be achieved in this way (whether it had anything to do with the origin of petroleum is another matter.) His experiments were repeated in Germany by Carl Engler and their publications also inspired some activities by other petroleum chemists. The technique of Day and Engler somewhat resembled chromatography, however, it was a kind of dead-end street, and there was no cross-influence between their work and Tswett's. Still, we can consider their approach as a forerunner of chromatography. Their activities are the subject of our present discussion.
David T. Day
David Talbot Day (1859–1925), a graduate of The Johns Hopkins University, Baltimore, Maryland, was associated from 1885 on with the U.S. Geological Survey, Washington, DC. First, he pioneered in systematizing information on the mineral resources of the U.S. Then, in the 1890s, he turned his attention to various aspects of the newly emerging petroleum industry. Finally, in 1920 he established a private laboratory in California, investigating the possibilities of recovering oil from shales.
By the last decade of the 19th century, oil production in Pennsylvania and Ohio was well established. Although chemical investigation of petroleum was still in its infancy, relying mainly on measuring its physical characteristics, it was noted that the characteristics of crude oils from different locations are different: one is heavier and contains more higher-boiling components than the other. Various explanations have been suggested for this difference, among them the so-called filtration hypothesis of Day, first described in a paper presented in 1897 [3]) He assumed that originally a primary oil existed at a certain location; this oil then migrated through various rock formations with help of diffusion. The various strata retarded more or less of the heavier fractions and, thus, the oils which passed through them and then accumulated at their final locations had different compositions.
In 1900, a famous international exposition (forerunner of present-day world's fairs) was held in Paris, France, and there, the U.S. had a representative pavilion with a special display of the American petroleum industry: Day was in charge of this exhibit. In conjunction with the exposition, the First World Petroleum Congress was held in August and there, Day presented a paper on his hypothesis. Its title was "The variation of the characteristics of crude oils from Pennsylvania and Ohio" (4). After describing the characteristics of different samples, he mentioned that he also carried out some "filtration experiments" by conducting crude oil through fuller's earth, resulting in some fractionation: the lighter fractions moved faster, before the heavier fractions, and this process imitates the movement of oil in the earth. However, he apparently did not give any actual data, nor did he describe the experimental setup (at least it is not included in the published text of his paper). After referring to a "German scientist" (most likely to Engler, but no name was given) who stated that it would be important if somebody could develop a method enabling the separation of individual hydrocarbons present in the crude oil, Day made the often quoted (actually misquoted) statement that "the filtration method offers good hope . . . [and] I believe that before the next winter, I will be able to accomplish complete separation." (Translation of the text of his lecture published in French.)
We do not know whether Day's statement reflected his optimism or an anticipation of future results. However, the realization of this hope definitely was beyond his capabilities and it took decades until his expectation could finally be accomplished by a different generation of petroleum chemists.
In the years following the Paris lecture, Day carried out some additional investigations but did not publish them; we only know of a lecture presented in 1903 at a meeting of the Geological Society of Washington, DC (briefly reported by Mendenhall [5]). In the following decade, he had two more papers: one co-authored with Gilpin (6) and a more detailed paper in which he extended his theory to the oils of California, Louisiana, Mexico, Oklahoma, Texas, and Wyoming, but again, without actual data (7). Finally, in 1922, Day published a handbook for the petroleum industry (8) and in it, he briefly discussed adsorption processes; as an illustration, he presented a table summarizing the results of Gilpin and Cram (9). Otherwise, however, he did not deal anymore with such investigations.
J.E. Gilpin
As mentioned earlier, Day was a graduate of The Johns Hopkins University and apparently he maintained some contact with his former alma mater, serving as an advisor in some research projects carried out by Professor Joseph E. Gilpin (1866–1924) and his graduate students. I know of three research papers by Gilpin's group published in 1908–1913 (9–11). These give a very detailed report of their investigations, with dozens of tables. However, they never achieved any real separation of individual components but measured only the physical constants of the individual fractions. Table I presents the results of one typical experiment (9).
The papers of Gilpin and associates also described the system used in the investigations and we can assume that essentially it was the same as originally used by Day (Figure 1). Glass or tin tubes of 3–6 ft length and 1–1.25 in. diameter. The fraction character did not translate properly.]were filled with fuller's earth, and these were placed vertically in open dishes containing the petroleum samples. The sample ascended in the tube very slowly: the experiment reported in Table I took one full day but even longer times were not unusual. The experiments might have been accelerated by applying vacuum to the top of the tubes (see Figure 1). At the end of the experiment, the oil-laden filling of the tubes was slowly and carefully removed stepwise and cut to fractions, and the oil in the fuller's earth fractions was removed by water displacement. The oil recovered in this way represented only a fraction of the original sample: about 40% remained permanently adsorbed on the fuller's earth packing. (It should be noted that the so-called fuller's earth has served at that time as the most widely used adsorbent, both in the industry and laboratory work. It consists mainly of hydrated aluminum magnesium silicate and its name reflects one of the main industrial uses by textile workers — the so-called fullers — to remove grease and oils from cloth. In the U.S., the most important fuller's earth deposits were in Florida. Therefore, the material also was called Florida earth.)
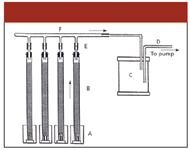
Figure 1: Experimental setup used by Gilpin and associates (10). The crude oil samples were placed in tin cans of about 1-L volume (A). The glass or tin tubes filled with fullerôs earth (B) were 3â6 ft long with 1â1.25 in. diameter. To accelerate the upward movement of the oil, sometimes vacuum (D) was applied to the top of the tubes through a manifold (F) and a large buffer volume (C); the manifold was connected to the tubes by small rubber tubes fitted with pinchcocks (E).
We can conclude that Day's prediction in his Paris lecture never materialized, and neither he nor Gilpin ever separated individual hydrocarbons. In fact, the actual aim of their investigations — and this is clear when reading their papers — was only to prove the validity of the "filtration hypothesis," that some fractionation could have happened during passage of crude oil from one place to the other.
Carl Engler
Carl Engler (1842–1925) graduated from the Technical College (later Technical University) of Karlsruhe, Germany, and from 1876 until his retirement in 1919, he was associated with this school as a professor of chemical technology. He was instrumental in developing this university as one of the most important European technical schools of higher learning. For four decades Engler was the most important German scientist involved in petroleum research, with significant contributions to both technology and testing: even today we speak about the Engler viscosimeter and Engler distillation, and his five-volume petroleum handbook has served for decades, even after his death, as the bible of chemists, engineers, and geologists.
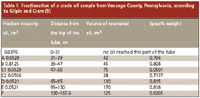
Table I: Fractionation of a crude oil sample from Venango County, Pennsylvania, according to Gilpin and Cram (9)
Engler apparently was present in Paris at Day's lecture and was very interested in his "filtration hypothesis." Therefore, after returning home, he started systematic investigations to study the process and to see whether adsorbents other than fuller's earth would also give similar results. Because Day gave no description of his experimental setup, Engler had to devise his own (Figure 2a). In Engler's system, the upward travel of the oil was facilitated by gravimetric pressure; also, he let the whole oil pass through the tube and collected portions of the effluent emerging from the top of the tube. He also devised another setup (Figure 2b) in which the fractions could be sampled at various heights of the tube filled with the adsorbent. In this way, the distribution of the individual fractions along the tube could be checked.
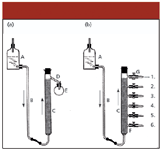
Figure 2: Experimental setup used by Engler and Albrecht (12). The oil sample was flowing from a reservoir (A) by gravimetric pressure through a glass connecting tube (B) to the bottom of the tube filled with fullerôs earth (C). The tubes were 80â90 cm (2).
Engler, together with E. Albrecht, published his results in 1901 (12). Similar to Day (and later Gilpin), he recorded only the changes in the physical characteristics of the individual fractions, without any attempt at further separation of individual compounds. Table II presents typical results of Engler and Albrecht (12). In addition to fuller's earth, they also tried other adsorbents ("various sands") but the results were essentially the same. They also carried out experiments with other samples — for example, a 1:1 (v/v) ethanol–aniline mixture — but again, only the specific weights of the individual fractions were reported.
Engler also considered the theoretical background of the (partial) fractionation occurring on fuller's earth. Apparently influenced by the then fashionable "capillary analysis" of Goppelsroeder (see, for example, the discussion in reference 13), he attributed the partial separation of the lighter fractions to capillary action. It took almost a decade until Ubbelohde clarified that fractionation on fuller's earth is due to selective adsorption and has nothing to do with capillary action (14).
The system of Engler and Albrecht clearly was more advanced than the one used by Day and Gilpin: it was faster and collection of the effluent was more convenient than the tedious work of dividing the fuller's earth packing into fragments and recovering the oil from these. Because the activities of Gilpin and his graduate students came seven years after Engler's publication, one might ask the obvious question, why did Gilpin not adapt Engler's system for his own investigations? Most likely the reason for this was that Gilpin's primary aim was (just as that of Day) to investigate changes occurring during passage of oil in the strata (modeled by the fuller's earth) through various geological formations, and not obtaining individual compounds. Another question one might ask is: because Gilpin's publications followed Tswett's, why did he not cite him? Most likely Gilpin was not aware of Tswett's work, but even if he had known about it, he would not consider any relationship to his investigations.
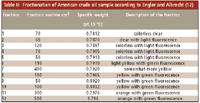
Table II: Fractionation of American crude oil sample according to Engler and Albrecht (12)
Other Scientists
We do not know of any further work of Engler along this line, however, a few petroleum chemists reported on some follow-up investigations. We should briefly mention three here.
Leo Ubbelohde
Leo Ubbelohde (1876–1964) studied at the University of Berlin, Germany, and from 1907 on (from 1910 on as a professor) was associated with the Technical University of Karlsruhe, Germany. In 1933, he moved to the Technical University of Berlin–Charlottenburg. He had been very active in associations in the field of petroleum research and production. From 1907 to 1914, he served as the Secretary General of the International Petroleum Commission and in 1933 he established the German Society for Mineral Oil Research. He was also active in the fields of textiles and natural fats. We are interested here in one of his papers from 1909 criticizing a paper of Rakusin. In this paper, he stated among others, that the theoretical basis of the "filtration experiments" of Day and Engler had nothing to do with capillary action but was based upon selective adsorption (14).
Russian Petroleum Chemists
Commercial exploitation of the oil deposits along the Caspian Sea, near Baku (in present-day Azerbaijan) began in 1872 and by the beginning of the 20th century it represented the largest oil field in the world. (Today, it is practically unknown that these fields were developed by brothers of Alfred Nobel and the income from oil production significantly contributed to the immense wealth of the family.) A number of important Russian chemists have been associated with the petroleum industry and they also carried out intensive research. However, although they published in well-known Russian and foreign scientific journals, a historical evaluation of their activities is still missing. Essentially, we know about them only from the review papers of Herbert Weil (15–17) and the discussion of Camin and Raymond (20).
With respect to our subject, the work of two Russian scientists should be mentioned: they are M.A. Rakusin and V.E. Herr. Both followed the investigations of Day and Engler, studying the fractionation of crude oil by "filtration" through fuller's earth. M.A. Rakusin (18) also believed that capillary action served as the basis of this process, and Ubbelohde's quoted paper (14) is a polemic discussion of this incorrect interpretation (and some other statements of Rakusin). The other Russian chemist is V.E. Herr who, in 1909, published a paper in the German petroleum journal on "contribution to the filtration of crude oils from Baku through fuller's earth" (19). In this paper, he concentrated on the possibility to eliminate the high-molecular-weight aromatic fraction of crude oil by selective adsorption. It is of interest to quote his statement that his investigations clearly showed that "fractionation in the usual sense (i.e., separation of individual components) does not take place."
Controversy (I)
In 1927, the American Petroleum Institute started its Project No. 6, with the aim of separation and identification of the individual chemical constituents of petroleum and petroleum products, a project lasting for 40 years (13,20). In this project, fractionation on silica gel was first used, followed by increasingly sophisticated methods and the work of Day, Gilpin, and others achieving only limited fractionation was slowly forgotten. After some decades, László Zechmeister, one of the pioneers of (classical) liquid chromatography, finally brought back their work from oblivion. In a lecture on the "History, Scope and Methods of Chromatography" presented at the Chromatography Conference of the New York Academy of Sciences held in November, 1946, he discussed among others the origin of chromatography and the activities of Tswett as the inventor of the technique. He also mentioned the investigations of Day and Gilpin and correctly stated that they should be considered as forerunners of chromatography (21). Zechmeister repeated this discussion in the introduction of his book published in 1950 (22).
Quite unexpectedly, just a few months after the publication of this book, a polemic article was published in Nature by Herbert Weil and Trevor I. Williams (23), criticizing Zechmeister that he was unjust to Day by naming Tswett as the inventor of chromatography. According to them, this distinction belongs to Day. Soon after this brief article, the first of Weil's series on the history of "industrial petroleum chromatography" was also published (15) and in it, he further elaborated on this question. In these papers, Weil and Williams falsely interpreted Day's 1900 lecture in Paris, claiming that in it, some form of chromatography had been described in detail. Therefore, they stated that August 20, 1900 (the day of Day's lecture in Paris) should be considered as the "birthday of chromatography."
Zechmeister politely tried to answer Weil and Williams, emphasizing that instead of diminishing Day's merits, he actually resurrected it from oblivion (24). However, Weil and Williams continued this polemics, repeating their claim that Day "did in fact discover and clearly describe a system of adsorption chromatographic analysis" (25). Of course, we have seen in the previous discussion that Day did not describe his system (this was done only eight years later by Gilpin) and that neither he nor his followers attempted to separate individual hydrocarbons. The papers of Weil and Williams clearly represented a misquotation, or misinterpretation, of Day's lecture.
Very wisely, Zechmeister stopped this polemic and did not continue to argue with Weil and Williams. Today, the role of Tswett as the true inventor of chromatography is universally recognized; at the same time, however, we should also recognize Day and Engler as forerunners, first pointing out the possibility of fractionation of petroleum by selective adsorption. Their work, using Zechmeister's words, "might have developed into systematic chromatography" (21).
Controversy (II)
The polemics between Zechmeister and Weil and Williams seemingly ended with the polite answer of Zechmeister. However, this is only true about western literature: Soon, the priority of the invention of chromatography was blown up to a political question, proof or denial of the achievements of the Soviet Union. In the January 2006 issue of our magazine, we shall deal with this second controversy which could be characterized as a milestone in human stupidity.
Acknowledgments
In the past, I have published two detailed discussions of the activities of Day, Gilpin, Engler, and associates: as part of a book chapter (13) and as an article in Chromatographia (26). The figures and tables included in the present article are from the latter. I would like to express my appreciation to the publisher of Chromatographia for permission to use this material.
Leslie S. Ettre From 1988 to 2004, "Milestones in Chromatography" editor Leslie S. Ettre was associated with the Chemical Engineering Department of Yale University (New Haven, Connecticut), first as an adjunct professor and then as a research fellow. Previously, he had been with the Perkin-Elmer Corporation for 30 years. He is currently a member of LCGC's editorial advisory board.
References
(1) L.S. Ettre, LCGC 21, 458–467 (2003).
(2) H.H. Bussemas and L.S. Ettre, LCGC 22, 262–271 (2004).
(3) D.T. Day, Proc. Amer. Philosophical Soc. 36, 112â115 (1897).
(4) D.T. Day, "La Variation des Caractères des Huils Brutes de Pennsylvania et de l'Ohio." Proc. Cong. Int. du Pétrole, Notes, Memoires et Documents. Journal de Pétrole, Paris, 1902; Vol. I, pp. 53–56.
(5) W.C. Mendenhall, Science 17, 1007–1008 (1903).
(6) D.T. Day and J.E. Gilpin, Ind. Eng. Chem. 1, 449–455 (1909).
(7) D.T. Day, Trans. Amer. Inst. Mining (Metal.) Engrs. 44, 219–224 (1911).
(8) Handbook of Petroleum Industry, D.T. Day, Ed. (Wiley, New York, 1922).
(9) J.E. Gilpin and M.P. Cram, Amer. Chem. J. 40, 495–537 (1908).
(10) J.E. Gilpin and O.E. Bransky, Amer. Chem. J. 44, 251–303 (1910).
(11) J.E. Gilpin and P. Schneeberger, Amer. Chem. J. 50, 59–100 (1913).
(12) C. Engler and E. Albrecht, Z. Angew. Chem. 14, 889–892 (1901).
(13) L.S. Ettre, "Evolution of Liquid Chromatography" in HPLC — Advances and Perspectives, vol. 1, Cs. Horváth, Ed. (Academic Press, New York, 1980), pp. 1–74.
(14) L. Ubbelohde, Petroleum (Berlin) 4, 1394–1397 (1909).
(15) H. Weil, Petroleum (London), 14, 5–12, 16 (1951).
(16) H. Weil, Petroleum (London), 14, 205–210 (1951).
(17) H. Weil, Petroleum (London) 15, 9–12, 18 (1952).
(18) M.A. Rakusin, Petroleum (Berlin) 5, 760 (1910).
(19) V.F. Herr, Petroleum (Berlin) 4, 1284–1287 (1909).
(20) D.L. Camin and A.J. Raymond, J. Chromatogr. Sci. 11, 625–638 (1973).
(21) L. Zechmeister, Ann. N.Y. Acad. Sci. 49, 145–160 (1948).
(22) L. Zechmeister, Progress in Chromatography 1938–1947 (Chapman & Hall, London), p. 3. (1950)
(23) H. Weil and T.I. Williams, Nature (London) 166, 1000–1001 (1950).
(24) L. Zechmeister, Nature (London) 167, 405406 (1951).
(25) H. Weil and T.I. Williams, Nature (London) 167, 906–907 (1951).
(26) L.S. Ettre, Chromatographia 40, 207–216 (1995).
Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Determining the Serum Proteomic Profile in Migraine Patients with LC–MS
April 17th 2025Researchers used liquid chromatography–mass spectrometry (LC–MS) in their proteomic analysis to compare the serum proteome of migraine patients with healthy controls and to identify differentially expressed proteins as potential migraine biomarkers.












