Determination of Psilocin and Psilocybin in Magic Mushrooms Using iHILIC®-Fusion and MS
The Application Notebook
Hallucinogenic mushrooms, known as magic mushrooms, contain psychoactive compounds such as psilocin and psilocybin (Figure 1). This hallucinogenic effect means they are constantly offered on the black market. Therefore, the reliable quantification of these compounds is a particularly important task for forensic analysis because their results have a significant impact on the judgement passed by the courts.
Hallucinogenic mushrooms, known as magic mushrooms, contain psychoactive compounds such as psilocin and psilocybin (Figure 1). This hallucinogenic effect means they are constantly offered on the black market. Therefore, the reliable quantification of these compounds is a particularly important task for forensic analysis because their results have a significant impact on the judgement passed by the courts.
Although there are many analysis methods available in forensic laboratories and in the scientific literature, the majority of them are based on reversed-phase liquid chromatography (LC) separation (1–6). Due to the highly hydrophilic nature of psilocybin and psilocin, reversed-phase LC is not able to provide sufficient retention for them. Moreover, it is crucial to develop new methods and techniques that can improve the analysis detectability, selectivity, and productivity. To fulfill these goals, the application of hydrophilic interaction liquid chromatography (HILIC) and mass spectrometry (MS) is investigated.
In this study, we aimed to use a charge modulated iHILIC®âFusion HILIC column for the analysis of extracts from hallucinogenic mushrooms and evaluate its potential for forensic application.
Experimental
LC–MS System: Agilent 1100 LC system and Bruker Esquire 6000 ion trap mass spectrometer, operated in positive ionization mode (ESI+). Chromatographic data were acquired and evaluated with ChemStation Rev. A. 10.02.
Column: 150 × 4.6 mm, 3.5-µm 100 Å iHILIC®-Fusion (P/N 110.154.0310, HILICON AB, Sweden)
Mobile Phase: 80:20 (v/v) acetonitrile–ammonium format (10 mM, pH 3.5)
Flow Rate: 0.5 mL/min
Column Temperature: 12 °C
Sample Preparation: Quasi-counter current extraction with methanol at 60 °C in a Shimadzu 10/A HPLC system. A 50-mg measure of airâdried and homogenized hallucinogenic mushroom was filled in the extractor chamber (an empty 250 × 4.6 mm HPLC column). The standard solutions were 5 µg/mL and 500 μg/mL for psilocin and psilocybin, respectively. Methanol was used as the solvent.
Injection Volume: 1 µL
Results and Discussion
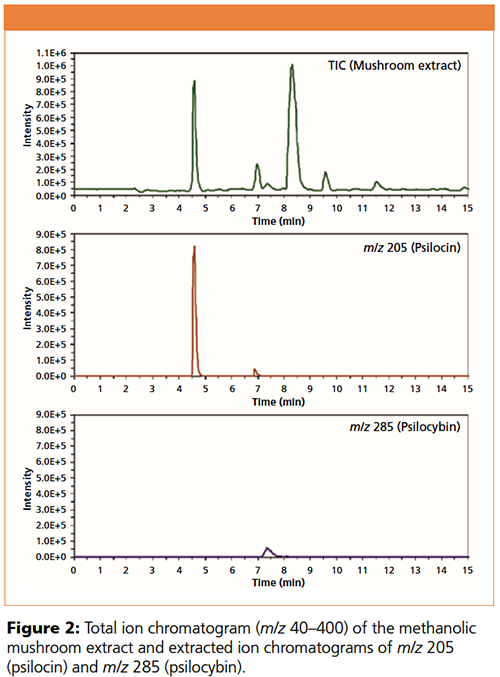
In our previous study (2), the methanolic mushroom extract was first separated under the conditions within a designed experimental space with a total of 18 model establishment points and two approval points, considering the mobile phase composition, pH, and temperature. The factors that affect the separation selectivity and resolution on three iHILIC® columns were studied using DryLab® and STATISTICA®. It was found that iHILIC®-Fusion provides best separation regarding separation selectivity and efficiency. Figure 2 illustrates the separation of mushroom extract and also the extract ion chromatograms at m/z 205 (psilocin) and m/z 285 (psilocybin), respectively. It is clear that iHILIC®-Fusion was able to separate psilocin and psilocybin from each other and also from the major matrix compounds within 15 min. An unique feature is that psilocybin elutes with a retention factor two times greater than that of psilocin. In addition, the sample preparation consists of few steps to minimize error sources and assure reliable results.
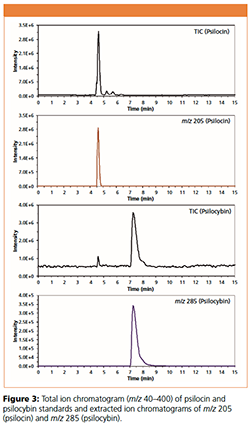
In the second step of this work, we separated the methanolic solution of psilocin and psilocybin standards to confirm the detection of these two alkaloids in the mushroom extract. As shown in Figure 3, both psilocin and psilocybin have identical retention times to the standards compared to those peaks from the mushroom extracts. Therefore, the developed method is selective for the two target compounds and can be used for the quantification as described in our early work (1).
Conclusion
This work illustrates how to use an iHILIC®-Fusion column and MS detection to separate and identify psilocin and psilocybin in hallucinogenic mushrooms or ”magic mushroom” extracts. This developed HILIC–MS method can be utilized in forensic and clinical applications.
References
- J. Nagy and T. Veress, J. Forensic Res.7, 356 (2016), DOI: 10.4172/2157-7145.1000356.
- N. Rácz, J. Nagy, W. Jiang, and T. Veress, J. Chromatogr. Sci. 57, 230–237 (2019).
- M.W. Beug and J. Bigwood, Journal of Chromatography207, 379–385 (1981).
- N. Anastos, S.W. Lewis, N.W. Barnett, and D.N. Sims, J. Forensic Sci.51, 45–51 (2006).
- R. Kysilka and M. Wurst, Planta Med.56, 327–328 (1990).
- V. Gambaro, G. Roda, G.L. Visconti, S. Arnoldi, and E. Casagni, J. Anal. Bioanal. Tech. 6, 277 (2015).

HILICON AB
Tvistevägen 48, SE-90736, Umeå, Sweden
Tel.: +46 (90) 193469
E-mail: info@hilicon.com
Website: www.hilicon.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



