Cracking the Code of Complex Drug Modalities via Multidimensional Liquid Chromatography Coupled to Mass Spectrometry
Multidimensional liquid chromatography, coupled to mass spectrometry (MDLC–MS) is a powerful tool for the characterization of complex biopharmaceutical drug modalities, from antibody–drug conjugates to nuclear acid therapeutics like antisense oligonucleotides and small interfering RNA.
Beyond just increasing peak capacity, multidimensional liquid chromatography (MDLC) methods, including an integrated bottom-up strategy, have revolutionized the characterization of complex drug modalities.
Bottom-up approaches are primarily used for two purposes: to sequence large molecules, and to profile impurities that cannot be separated or
detected at the intact molecule level. Bottom-up approaches are implemented in a good manufacturing practice (GMP) environment to confirm the sequence of therapeutic molecules. In practice, liquid chromatography–ultraviolet (LC–UV) profiles generated after an enzymatic digestion are typically compared to those obtained for a reference material to confirm the sequence for a new lot. The characterization of complex drug modalities is often performed at different molecular levels to provide a comprehensive impurity profile. For example, therapeutic antibodies are typically characterized at the intact level to quantify the charge and size variants, and also at the peptide level to quantify post-translational modifications (PTMs). Reducing the molecule complexity is achieved by reducing the molecule size after enzymatic digestion to produce smaller fragments more amenable to LC separation and tandem mass spectrometry (MS/MS) sequencing.
However, conventional in-solution enzymatic digestion is time consuming (>1 h), and it requires a large sample amount (>50 μg). In addition, long digestion times may cause an artificial degradation of the sample (1). MDLC setups, including an online sample preparation step, streamline the characterization of complex drug modality impurities. The major advantages of using MDLC include being able to use a significantly reduced sample amount (<10 μg) and digestion time (<5 min) compared to conventional offline approaches. MDLC coupled with mass spectrometry (MDLC–MS) enables the high throughput screening of new drug candidates evaluated at an early stage and the extended characterization of impurity peaks separated by the reference LC methods at a later stage.
Antibodies
A plethora of MDLC–MS methods have been developed since Gstöttner and others reported the online fractionation of charge variants using first dimension (1D) cation exchange (CEX) chromatography and further determining PTMs by online peptide mapping (2,3). The identification and quantification of PTMs for fractionated charge variants has attracted more attention, and several groups have reported on them (1–4). The four-dimensional (4D)-LC–MS analytical workflow is illustrated in Figure 1.
FIGURE 1: Schematic representation of the 4D-LC–MS setup for the automated characterization of antibody charge variants by an online peptide mapping procedure, enabling the separation and fractionation of charge variants by ion exchange chromatography (IEX) (1D) followed by on-column reduction by RPLC (2D), trypsin digestion of the light chains (LC) and heavy chains (HC) in flow-through mode (3D), and peptide mapping analysis by RPLC (4D). Reproduced from (2) with permission from the Royal Society of Chemistry, UK.
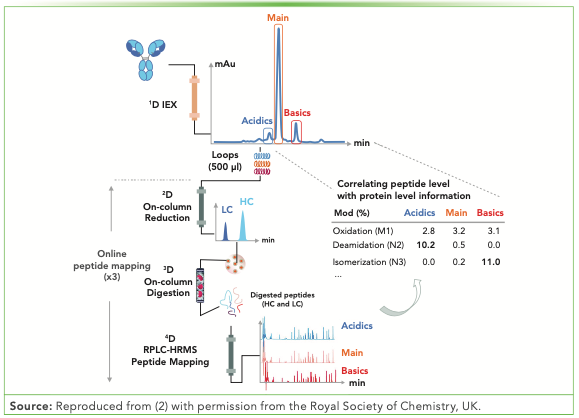
Similar sequence trypsin coverage and oxidation or deamidation levels have been observed when comparing the online and offline approaches (1). An interlaboratory study demonstrated the successful application of MDLC–MS from multiple sites (5).
Non-denaturing CEX chromatography, size-exclusion chromatography (SEC), hydrophobic interaction chromatography (HIC), and protein A modes can be easily coupled to reversed-phase LC (RPLC) because of the high aqueous conditions, enabling the versatile 4D-LC–MS systems with the use of alternative modes to 1D CEX, such as SEC or Protein A (6,7). For example, the average drug-to-antibody ratio (DAR) and PTMs were determined for the aggregates separated from the monomeric peak of an interchain cysteine-linked antibody–drug conjugates (ADC) by SEC (6). The results suggested that the aggregation mechanism primarily involved antibodies with their heavy chains (HCs) conjugated to two or three linker drugs (6). MDLC–MS can also be implemented as an in-process control (IPC) method, which is demonstrated by the identification and quantification of antibody PTMs for fractions collected from cell cultures (7). The antibody was separated from host cell proteins using 1D protein A chromatography and the PTMs were characterized using a 4D-LC–MS method (7).
Nucleic Acids
An acceleration in the approval of antisense oligonucleotide (ASO) and small interfering RNA (siRNA) therapeutics by regulatory agencies has been observed over the last five years (8). ASO and siRNA have molecular weights typically between 5–15 kDa. Their length is amenable to full sequencing by MS/MS, and N-1 or N+1 impurities can be chromatographically resolved (8). Advances in biology, oligonucleotide chemistry, and drug delivery systems have led to the development of larger nucleic acids, such as messenger RNAs (mRNA) and guide RNAs (gRNA). The gRNAs contain approximately 100 nucleotides, whereas mRNA consists of hundreds to thousands of nucleotides. The full sequencing of gRNAs and mRNAs by top-down MS has not been reported yet. Conversely, bottom-up approaches involving parallel digestion have been developed to sequence large RNA molecules and gRNAs (9,10).
To our knowledge, for the first time in 2021, the online digestion and separation of an RNA molecule was reported (11). A 2D-LC approach was developed to sequence gRNA molecules via 1D parallel ribonuclease (RNase) digestion and 2D hydrophilic interaction liquid chromatography (HILIC) separation prior to MS/MS sequencing (Figure 2).
FIGURE 2: Schematic representation of the automated 2D-LC workflow for parallel RNase digestions coupled to HILIC. The HILIC profiles obtained after RNase T1, A, and U2 digestions for sgRNA A (I) and sgRNA B (II) are represented by the blue, grey, and green traces, respectively. Reproduced from (11) with permission from the American Chemistry Society.
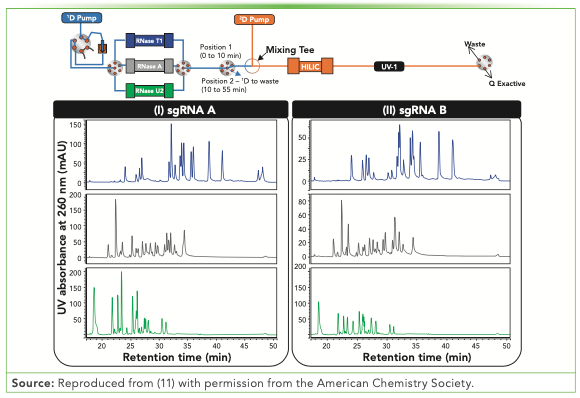
A 10-fold reduction in the gRNA amount and digestion time was achieved by using an online approach instead of a offline approach. The sequence coverage obtained after RNase T1 digestion were the same (11). The RNase T1-HILIC 2D-LC sequencing procedure can be further coupled to a reference separation technique. For example, fractions collected from 1D ion-pairing RPLC (IPRP) can be characterized at the single nucleotide level by RNase T1-HILIC. The three-dimensional (3D)-LC–MS approach can potentially reveal impurities that cannot be separated and detected at the RNA intact level.
Conclusion and Future Opportunities
In this study, the utility of MDLC–MS has been demonstrated for real-world project support. The broader use of MDLC–MS technology could be accelerated by developing user-friendly software interfaces to facilitate the implementation of MDLC workflows, additional immobilized enzyme cartridge for antibodies and oligonucleotides, and ready-to-use reactors for denaturing drug delivery systems or chemically reducing protein species.
Drug delivery systems, such as lipid nanoparticles, have contributed to the success of the mRNA vaccines developed for the 2019 coronavirus disease (Covid-19). These complex and large systems have to be characterized at the particle and molecular levels. For example, the nanoparticle size and free drug concentration are determined at the particle level, whereas the encapsulated drug and lipids forming the layer are commonly characterized at the molecular level after denaturing the lipid nanoparticle (LNP) via a surfactant.
Therefore, MDLC–MS setups present a formidable opportunity to unify the characterization of drug delivery systems at the molecular and particle levels, which would enable their high throughput analysis.
Acknowledgments
I would like to thank Brandon Scott, Dr. Kelly Zhang, and Dr. Pete Yehl of Genentech for the review and suggestions.
References
(1) A. Goyon, L. Dai, T. Chen, B. Wei, F. Yang, N. Andersen, et al, J. Chromatogr. A. 1615, 460740 (2020).
(2) J. Camperi, A. Goyon, D. Guillarme, K. Zhang, and C. Stella, The Analyst 146, 747–769 (2021).
(3) C. Gstöttner, D. Klemm, M. Haberger, A. Bathke, H. Wegele, C. Bell, and R. Kopf, Anal. Chem. 90, 2119–2125 (2018).
(4) L. Verscheure, A. Cerdobbel, P. Sandra, F. Lynen, and K. Sandra, J. Chromatogr. A. 1653, 462409 (2021).
(5) J. Camperi, I. Grunert, K. Heinrich, M. Winter, S. Özipek, S. Hoelterhoff, et al, Talanta 234, 122628 (2021).
(6) A. Goyon, M. Kim, L. Dai, C. Cornell, F. Jacobson, D. Guillarme, and C. Stella, Anal. Chem. 91, 14896–14903 (2019).
(7) J. Camperi, L. Dai, D. Guillarme, and C. Stella, Anal. Chem. 92, 8506–8513 (2020).
(8) A. Goyon and K. Zhang, Anal. Chem. 92, 5944–5951 (2020).
(9) T. Jiang, N. Yu, J. Kim, J.-R. Murgo, M. Kissai, K. Ravichandran, et al, Anal. Chem. 91, 8500–8506 (2019).
(10) A. Goyon, B. Scott, K. Kurita, C.M. Crittenden, D. Shaw, A. Lin, et al, Anal. Chem. 93, 14792–14801 (2021).
(11) A. Goyon, B. Scott, K. Kurita, C. Maschinot, K. Meyer, P. Yehl, and K. Zhang, Anal. Chem. 94, 1169–1177 (2022).
ABOUT THE AUTHOR
Alexandre Goyon is with the Small Molecule Analytical Chemistry department at Genentech Inc., in South San Francisco, California. Direct correspondence to: goyon.alexandre@gene.com


New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








