Column Plate Number and System Suitability
LCGC Asia Pacific
How suitable is the column plate number for system suitability testing?
John W. Dolan, LC Troubleshooting Editor.
How suitable is the column plate number for system suitability testing?
I was recently contacted by a colleague inquiring about whether or not the column plate number, N, is a good measurement to include in system suitability testing (SST) for liquid chromatography (LC) methods. He had noticed that monographs in the United States Pharmacopoeia (USP) often had minimum plate number requirements as part of SST for those methods, but that often it was specified that N > 2000 was sufficient. He wondered about the origin of a N > 2000 requirement and if it really added any value to SST, especially in light of the performance of modern LC columns. This is a topic of wide enough interest that I feel it is worth a discussion here.
Background
The column plate number usually is part of the specification for new columns. Traditionally, N is calculated based on manual measurements of the retention time, tR, and the baseline width, wb, measured between tangents to a chromatographic peak, as shown in Figure 1 using the formula
N = 16(tR/wb)2 [1]
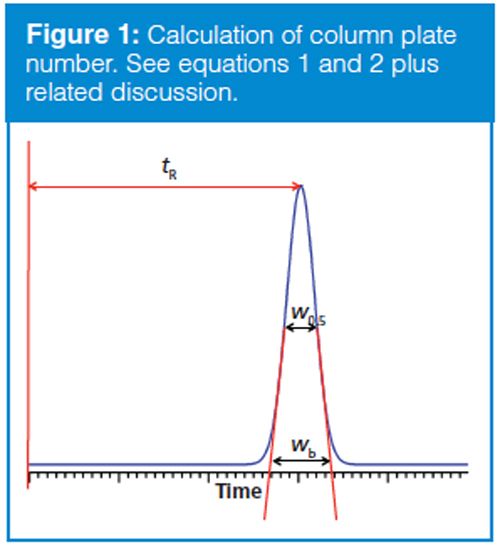
More commonly today, N is measured by data systems based on the more easily measured width at half the peak height, w0.5, using
N = 5.54 (tR/w0.5)2 [2]
The method of equations 1 or 2 only applies to isocratic separations, where the mobile-phase composition is constant, and should not be used for gradient separations.
A well-packed, reversed-phase column containing totally porous particles tested under ideal conditions should generate plate numbers of
N ≈ (1000L)/(2dp) [3]
where L is the length of the column (in millimetres) and dp is the particle diameter (in micrometres). However, this requires an LC system with very low extracolumn volume, a small injection, a well-behaved, small molecular weight test solute, an optimum flow rate, and other conditions that do not reflect typical application of the column in the routine laboratory. Also, if equation 3 were used for acceptance criteria of a new column, column manufacturers would have to discard many otherwise satisfactory columns. As a result, equation 3 may be adjusted to use 2.5 dp (or some other value) rather than 2 dp as the denominator for a quality control specification in the column manufacturing process.
When an LC column is used for a real LC method, usually the conditions are much less ideal than those used by the manufacturer for testing the column. The typical LC system will have more extracolumn volume than the test system. The sample solutes will be less wellâbehaved, and other chromatographic conditions (temperature, flow rate, and so forth) may also degrade the column performance. It is nice to have a rule of thumb for column performance under typical, “real” conditions to make sure the column is performing adequately. For totally porous particle columns I like to use the estimate
N ≈ (300L)/dp [4]
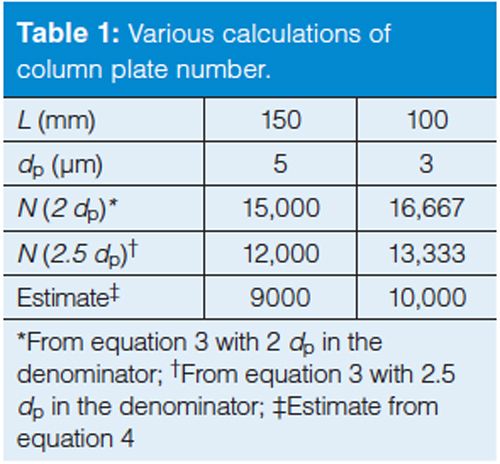
In Table 1, I have compared the calculated plate numbers for two of the most popular column configurations today: a 150-mm, 5-µm dp column and a 100-mm, 3-µm column.
Some Practical Uses of N
Let’s consider some of the ways the knowledge of the plate number for a particular column can be useful. You can see from Table 1 that the plate number for the 150 mm, 5 µm column is approximately the same as the 100 mm, 3 µm one. This means that these columns should give the same separation if the same column chemistry (brand and product line) and operating conditions are used. This can be helpful in evaluating tradeoffs when changing column dimensions - if the 150-mm column had a 15-min run time, it would only take 10 min with the 100âmm column and would reduce solvent consumption by a third.
You can also monitor the column performance over time for help in deciding when to replace the column. I usually consider that the column should be replaced when you see ~30% loss in N relative to the value measured when the column is new. So, if you measured N = 9000 for a new column and your method, when it dropped to N ≈ 6000, it probably would be time to replace it. The replacement decision should also be based on other factors, such as changes in peak shape and back pressure.
The column plate number can be helpful in evaluating overall performance of the LC system. If you suspect that the cause of a drop in plate number is external to the column, you can replace the column with a new one and compare the plate number (and other performance parameters) to historic values when the system was known to be operating well. If N is still low with a new column, look for problems external to the column. Extracolumn band broadening can be a critical factor in such cases, especially with the increased popularity of ultrahigh performance LC (UHPLC), where 50 mm × 2.1 mm columns packed with ≤2-µm diameter particles are popular. A fitting that has slipped a bit because of system over-pressure or poor assembly is not visible to the naked eye, but often will cause noticeable deterioration of chromatographic performance, including N.
Resolution
Resolution, Rs, is another parameter that is used to evaluate the suitability of a method for analysis. It is useful to compare the utility of N and Rs and their roles in system suitability, so let’s take a slight detour and review how we determine resolution.
Resolution can be calculated using the retention times of the two peaks (tR1 and tR2) and the peak widths at baseline (wb1 and wb2):
Rs = (tR2 – tR1)/(0.5(wb1 + wb2)) [5]
Alternatively, the widths at half-height (w0.5,1 and w0.5,2) can be used:
Rs = (tR2 – tR1)/(1.7 × 0.5(w0.5,1 + w0.5,2)) [6]
Rs = (1.18 ((tR2 – tR1))/(w0.5,1 + w0.5,2) [7]
As with the calculations of N at half the peak height, equation 6 (or 7) is a simpler measurement for the data system than peak widths at the baseline. A further advantage of the half-height technique is that if Rs < 1.5, baseline peak widths will be difficult or impossible to determine even for well-shaped peaks, so the method of equation 5 cannot be used. As you can see from equations 5, 6, and 7, resolution is directly related to the peak width.
Another way to determine resolution is with the fundamental resolution equation:
Rs = 0.25N0.5(α – 1)(k/(1 + k)) [8]
where k is the retention factor and α is the selectivity, calculated as the ratio of retention factors for two adjacent peaks. Values of k, and thus α, are controlled by the chemistry of the mobile and stationary phases as well as the temperature. So, if chemistry and temperature are constant, as is normally the case, resolution is a function of the square root of the plate number.
N as a System Suitability Parameter
Often the column plate number is included as part of system suitability testing, such as the N > 2000 requirement mentioned in the introduction. If, as is the case most of the time, one goal of an LC method is to separate two compounds, the resolution (Rs) of the two peaks will be included as part of SST. If resolution measurement is required, does measurement of N add any useful information to SST?
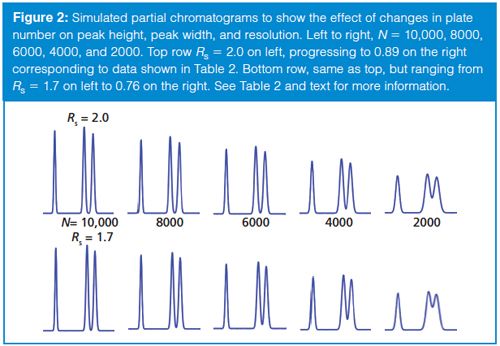
To help compare and contrast Rs and N, I’ve prepared Table 2 and Figure 2. Table 2 tabulates peak and chromatogram characteristics for different values of N. The various rows show data corresponding to columns with plate numbers ranging from 10,000 down to 2000 in 20% increments (data in columns 1 and 2). This progression might occur when we start with a new, 150-mm, 5-µm dp column (N ≈ 10,000 per Table 1) and watch the plate number drop over the lifetime of the column. If the chemistry of the system stays the same, the retention will stay constant, so decreasing N will increase the peak width as a square root function (see equations 1 or 2); the peak width is shown in the third column of Table 2. For constant sample size and detector response, the peak height will drop in proportion to the increase in peak width (column 4). As we saw above in equation 8, resolution will be reduced by the square root of the drop in N; resolution relative to the starting condition is shown in column 5. Notice that it takes a fourfold drop in N to halve Rs. Finally, columns 6 and 7 translate this relative loss of resolution into numeric values for separations starting with Rs = 2.0 or 1.7.
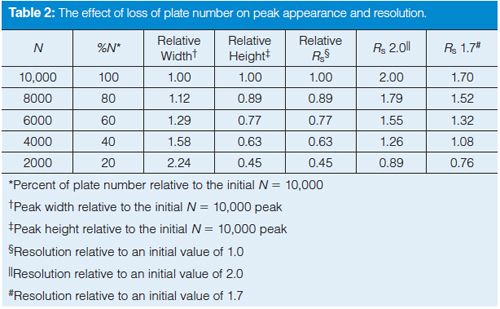
Figure 2 comprises simulated partial chromatograms based on the data in Table 2. In each chromatogram, a wellâresolved peak precedes a peak pair whose resolution is tracked as follows. The top row of chromatograms corresponds to column 6 of Table 2 where Rs = 2.0 initially (left) and drops progressing to the right as the plate number drops to N = 2000 and Rs = 0.89. The bottom row of Figure 2 shows the corresponding chromatograms starting with Rs = 1.7.
One thing that is quickly apparent when studying the chromatograms in Figure 2 is that a change in N may be less likely to be noticed than a change in Rs. For example, the loss of ~25% in peak resolution is obvious when comparing the chromatograms for N = 10,000 and N = 6000, whereas the loss of N, often noticed by increased peak width, is much less obvious without careful study. In Figure 2, the loss of peak height is obvious as N is decreased, but here all the chromatograms are on the same scale; if the peak heights were normalized to the tallest peak, a 25% change in peak height might be overlooked in a visual evaluation of the chromatogram.
Another problem with the plate number as a measure of quality is that the absolute value of the plate number does not necessarily reflect the quality of the separation. For example, compare the initial Rs = 2.0 separation after the column degrades to N = 6000 to the original Rs = 1.7 separation with the N = 8000 column. Both have the same resolution (Rs = 1.5), but N differs by 25%. Thus, setting some arbitrary minimum acceptable value of N, as was proposed in the introduction for N > 2000, makes no sense as a general requirement. Instead, the minimum acceptable plate number can be determined only in light of the minimum acceptable value of resolution for each method. On the other hand, a specified value of resolution will always translate into the same visual appearance of the chromatogram regardless of the method or the column plate number.
Does resolution always win out over the plate number as an effective SST parameter? Not necessarily. A value for resolution is of little practical use once two peaks are well separated at the baseline (for example, Rs > 3). This is apparent for the resolution between the first and second peaks of each partial chromatogram in Figure 2; in no case is a numeric value of resolution very useful in alerting us to potential problems with the separation. The plate number, on the other hand, does give us an idea that something is not going well when the value drops precipitously.
The plate number also gives us information regarding how well the column performs relative to an ideal case (manufacturer’s test), a generic rule of thumb for “good” performance (equation 4), or the same column when it was new. Tracking the plate number can help us determine when the column is nearing the end of its useful life so we can order a replacement. Following changes in the column plate number can also help to anticipate problems with detection limits. As the plate number drops, the width increases and the peak height decreases (Table 2) so that peak area remains constant. Whereas most of us use peak area for quantification, peak height is the critical measurement when it comes to detection limits, which depends on the signal-to-noise ratio. Shorter peaks will have smaller signal-to-noise ratios, and thus poorer detection limits. When methods are used for trace analysis, the plate number may serve as a very useful tool to help determine when to retire a column so as to maintain suitable lower quantification limits.
A final consideration for the plate number is reflected in the speed of separation. Larger plate numbers (for the same column length) always translate into narrower peaks. Narrower peaks allow peaks to be more closely eluted for any given resolution than for broader peaks. Therefore, larger plate numbers can be translated into faster separations. So the use of columns that generate substandard values of N will require longer run times and thus increase analysis costs.
Conclusions
We have looked at the column plate number as a system suitability parameter to include in LC system suitability testing. We have seen that resolution is much more useful than N if the peaks are close together (for example, Rs < 3), but its usefulness drops for very well separated peaks. Therefore, the plate number can be a useful way to predict method performance when the separation is not challenging. It can also help to determine the overall health of the column and its performance compared to some specified reference condition. Because both N and Rs can be calculated easily and automatically by chromatographic data systems, I think it is a good idea to keep track of both measurements. Just be sure to use the data appropriately to evaluate current or potential problems with an LC method. And an arbitrary lower limit of N > 2000? I don’t think this is a good idea.
So where did that N > 2000 requirement come from, anyway? As far as I can tell, it originated in a United States Food and Drug Administration (FDA) document (1). This document is designed to help FDA inspectors evaluate chromatographic methods when they conduct laboratory audits. In the guidance, the recommendation is, “The theoretical plate number depends on the elution time but in general should be >2000”. The document is dated 1994, and, although 5 µm dp columns were widely used by that time, older columns packed with 10-µm irregularly shaped particles were still common. There may have been some justification for N > 2000 20 years ago, but not today, and certainly not as a generic requirement.
Reference
- U.S. Food and Drug Administration, Reviewer Guidance. Validation of Chromatographic Methods (USFDA-CDER, Rockville, Maryland, USA, November 1994).
“LC Troubleshooting” Editor John Dolan has been writing “LC Troubleshooting” for LCGC for more than 30 years. One of the industry’s most respected professionals, John is currently the Vice President of and a principal instructor for LC Resources in Lafayette, California. He is also a member of LCGC Asia Pacific’s editorial advisory board.
Evaluating Body Odor Sampling Phases Prior to Analysis
April 23rd 2025Researchers leveraged the advantages of thermodesorption, followed by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC/TOF-MS), to compare and assess a variety of sampling phases for body odor.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








