Characterization of Allergens in Cosmetics by GC×GC–TOF-MS with Variable-Energy Electron Ionization
The Column
The detection and quantitation of allergens in complex cosmetics extracts using two-dimensional gas chromatography–time-of-flight mass spectrometry (GC×GC–TOF-MS) is described. In particular, variable-energy electron ionization is shown to enhance both the sensitivity and selectivity of analyses by generating mass spectra containing structurally significant fragment ions with an improved molecular ion signal.
The detection and quantitation of allergens in complex cosmetics extracts using two-dimensional gas chromatography–time-of-flight mass spectrometry (GC×GC–TOF-MS) is described. In particular, variable-energy electron ionization is shown to enhance both the sensitivity and selectivity of analyses by generating mass spectra containing structurally significant fragment ions with an improved molecular ion signal.
In 2003 a European Union Directive restricting the use of allergenic compounds in fragrances was released.1 The directive named a total of 27 allergens that should be labelled if present at over 100 ppm in "wash-off" products, such as shower gels, or more than 10 ppm in "leave-on" products, such as perfumes. To comply with this directive, accurate detection and quantitation methods are required, and two-dimensional gas chromatography–time-of-flight mass spectrometry (GC×GC–TOF-MS) is well-suited. The coupling of two columns of different stationary phases minimizes the requirement for sample preparation steps that can introduce error into the analytical process.

Photo Credit: Tetra Images/Getty Images
Despite this, the identification of individual compounds in complex samples remains challenging when multiple compounds in a chemical class have similar spectra, or weak molecular ions. This can be addressed with the use of soft ionization to reduce the degree of ion fragmentation, but this can be cumbersome to implement.
In this article, the use of GC×GC–TOF-MS to identify allergens is described. A recently developed variable-energy ion source technology is demonstrated to enable efficient electron ionization at lower energies, resulting in improved compound identification and detection limits.
Methods
A series of calibration standards containing allergenic compounds were prepared in acetone, ranging in concentration from 0.2 ppm to 10 ppm. Two cosmetics extracts were also prepared, one from a cream and the other from a perfume. Further details of the calibration are available from the authors.
GC Conditions: The analysis was performed on a GC instrument (7890A, Agilent Technologies). Injector: Split/splitless injector; Liner: 4.0 mm i.d. liner, 1 μL injection; Carrier gas: Helium, constant flow at 1.0 mL/min; Mode: Split 10:1; Inlet temperature: 230 °C; Septum purge: On, 3 mL/min.
2D Column Set: 1st dimension: 30 m × 0.25 mm, 0.25 μm SolGelWax (SGE Analytical Science); 2nd dimension: 2.6 m × 0.1 mm, 0.1 μm DB1 (Agilent Technologies); Modulation loop: As for 2nd dimension; Column set: Equivalent pneumatic impedance to 35 m × 0.18 mm.
Temperature Programme: Main oven: 40 °C (1.0 min), 6 °C/min to 150 °C, 4 °C/min to 250 °C (20 min); Secondary oven: No offset; Hot jet: 140 °C (1.0 min), 6 °C/min to 210 °C, 4 °C/min to 250 °C; Cold jet: Dewar fill: high, 45%; low, 35%; Modulation period: 2.5 s, hot-jet pulse 350 ms; Total run time: 64 min.
MS Conditions: A BenchTOF-Select instrument (Markes International) was used for analysis. Filament voltage: 1.8 V; Ion source: 250 °C; Transfer line: 280 °C; Mass range: 40–600 amu; Data rate: 50 Hz with 200 spectra per data point. Image processing software: GC Image, LLC.
Results and Discussion
Compound Separation and Identification: A calibration series consisting of four standard solutions was analyzed using the conditions described. Separation of all 29 target compounds was achieved with the reverse-phase (polar–apolar) column set (Figure 1).
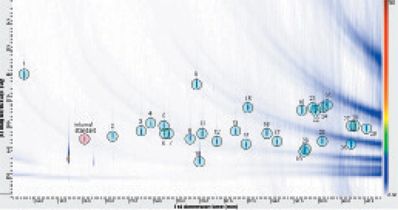
Figure 1: Close-up view of the GC×GC–TOF-MS contour plot of the allergens standard. The numerical labels refer to the compound identities in Table 1.
Spectral comparisons against the National Institute of Standards and Technology (NIST) database were performed for all allergens in the calibration mixture - this was possible because of the ability of the system to deliver "classical" EI spectra, with no mass discrimination. Retention times and library match results for all 29 target compounds are summarized in Table 1.
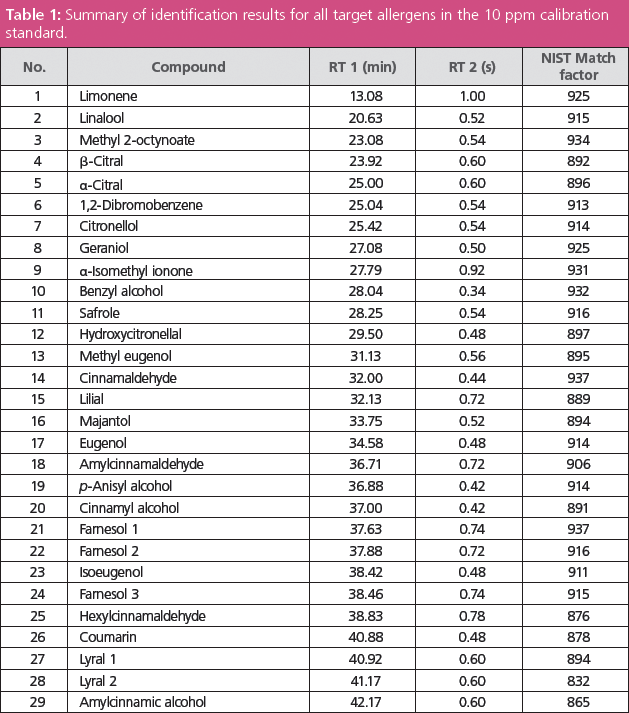
Table 1: Summary of identification results for all target allergens in the 10 ppm calibration standard.
Quantitation of Target Compounds: A template file containing qualifier expressions that had been prepared by the image processing software was used to automatically identify allergens in all samples, allowing fast processing of results. The calibration curves obtained were then used to quantify allergens present in the cream and perfume samples. Contour plots of the cream and perfume extracts are provided in Figure 2, with identifications and quantitative results annotated.
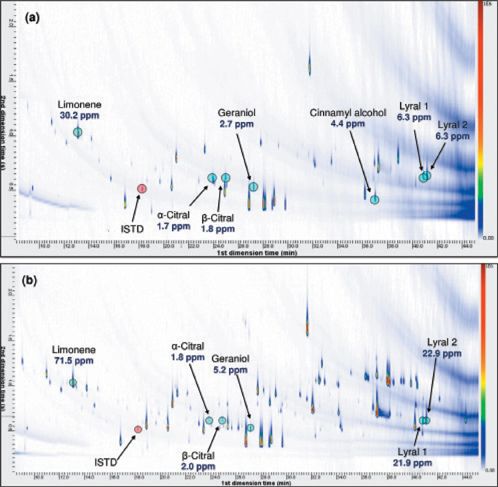
Figure 2: Quantified allergens in the (a) cream and (b) perfume extracts.
The cosmetics extracts analyzed contained fairly low background matrix because robust sample preparation techniques were used. However, it can be seen that the GC×GC–TOF-MS method provided the high degree of chromatographic resolution necessary to separate target compounds and potential interferences.
Variable-Energy Electron Ionization: Isomeric compounds commonly have similar mass spectra at 70 eV, making precise identification of individual isomers near impossible without the time-consuming analysis of standards to obtain retention time qualification.
However, the ability to generate electron ionization (EI) spectra at lower energies without a loss of sensitivity provides an extra dimension of information that allows the unambiguous identification of specific isomers. This is clearly demonstrated for the farnesol isomers in Figure 3.
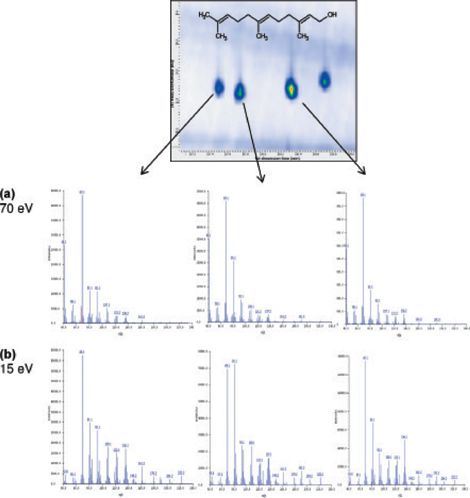
Figure 3: Spectra of three farnesol isomers at (a) 70 eV and (b) 15 eV. The greater degree of difference between the latter spectra aids identification.
This is particularly useful in GC×GC–TOF-MS analyses, where the added sensitivity and selectivity delivered by low-energy electron ionization complement the highly structured separation space occupied by extremely complex samples.
The stronger molecular ion and reduced fragmentation at lower ionization energies also improved signal-to-noise ratios and therefore detection limits, as shown in Figure 4 for safrole and coumarin.
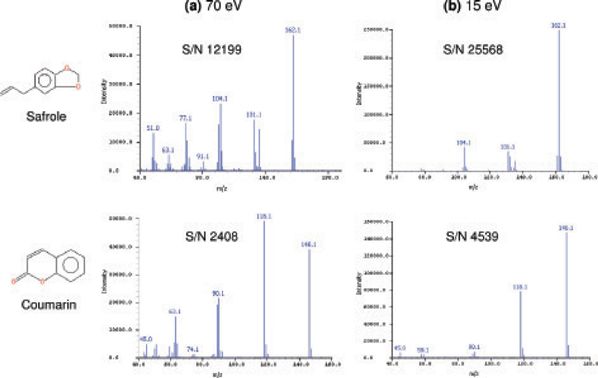
Figure 4: Spectra of safrole and coumarin at (a) 70 eV and (b) 15 eV. The reduced fragmentation at lower energies leads to a significant improvement in signal-to-noise ratios.
Conclusion
This article has shown that GC×GC–TOF-MS can provide a high degree of compound separation, sensitivity, and spectral quality in the analysis of allergens and other compounds in cosmetics extracts.
The value of variable-energy ionization technology to generate complementary spectra for enhanced compound identification is also demonstrated, along with an indication of the improved sensitivity and selectivity that this can offer.
Reference
1. Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products.
Stephen Smith studied in Bristol, UK, for both his B.Sc. and Ph.D., which he obtained in 2008 on innovative work profiling volatile organic compounds for disease diagnosis. Following post-doctoral positions at the University of the West of England and Bristol University, Steve joined Markes International as a senior applications specialist for thermal desorption and TOF-MS in 2011, where he now specializes in GC×GC–TOF-MS.
Laura McGregor received her M.Sc. in chemistry from the University of St Andrews, UK. She completed a further M.Sc. in forensic science at the University of Strathclyde, UK, followed by a Ph.D. in environmental forensics. Laura's research interests include the chemical fingerprinting of environmental contamination using advanced techniques such as GC×GC–TOF-MS. In 2013 she joined Markes International as a sales support specialist for their TOF-MS products.
David Barden is a technical copywriter at Markes International, having joined the company in 2011. David studied natural sciences at the University of Cambridge, UK, and remained there for his Ph.D. in organic chemistry, which he received in 2003. A placement at the European Journals Department of Wiley-VCH, Weinheim, Germany, was followed by seven years as a technical editor for various scientific journals at the Royal Society of Chemistry, Cambridge, UK.
E-mail: enquiries@markes.com
Website: www.markes.com

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).












