Bruker – Ultrahigh Sensitivity Proteomics on the timsTOF SCP
The timsTOF Pro platform, introduced in 2017, already featured a sensitivity boost from the Parallel Accumulation Serial Fragmentation (PASEF®) technology, which provides time-and-space focusing of the ions in the Trapped Ion Mobility Spectrometry (TIMS) tunnel. The timsTOF SCP platform further enhances the sensitivity with robustly modified ion optic design. This boost in sensitivity enables measurement of low nanogram and sub-nanogram peptide loads, resulting in quantification of a few thousand protein groups per injection. Our data from as low as 200 pg of peptide loads demonstrates the applicability to unbiased true single cell proteomics in a routine fashion.
Single-cell ‘omics in recent years has highlighted the microheterogeneity in clonal populations. Unlike other ’omics technologies, single-cell proteomics is hindered by lack of amplification techniques for protein molecules. Single-cell proteomics is now beginning to get attention, and one common strategy is to multiplex labeled single cells together with carrier samples to boost the sensitivity in detection. Further improvements in sophisticated sample preparation techniques have resulted in increased peptide yield that is delivered to the liquid chromatography–mass spectrometry (LC–MS) instrumentation (1). While these improvements upstream of sample measurement have improved the analysis depth, the raw sensitivity requires improvement as well without compromising robustness. Parallel Accumulation and Serial Fragmention (PASEF) (2) on the timsTOF Pro platform makes efficient usage of the ion beam and with intelligent precursor placement within a TIMS cycle achieves rapid-sequencing speed. In addition, the ions get focused in space and time within the TIMS cell, resulting in a significant boost in sensitivity. This enables the analysis of low sample amounts, in the range of low-nanogram peptide loads. The newly designed timsTOF SCP’s ion optics allow a 4–5× improvement in ion current by increasing the ion brightness while maintaining the robustness of the timsTOF Pro. As the yield of the electrospray ionization increases with lower flow rates, we further enhanced the experiment’s overall sensitivity by coupling the timsTOF SCP to an Evosep One (Evosep Biosystems) instrument operated with the new low-flow Whisper methods. We have characterized the resulting system’s performance by injecting and measuring peptide loads mimicking the amount resulting from a single-cell preparation.

Methods
The new ion optic design of the timsTOF SCP system includes switching the orientation of the ion optics with inclusion of an additional ion funnel and additional orthogonal turns of the ion beam to preserve the robustness of the instrument. The source contained a wider glass capillary orifice that draws more ions into an additional funnel housed in a multi-stage differentially pumped region. Our initial experiments demonstrated that in addition to the brighter ion beam these dedicated modifications were crucial to gain a factor five boost in ion current. For ultrahigh sensitivity measurements from 200 pg to a few nanograms of peptide, we coupled an Evosep One system (Evosep Biosciences) to the timsTOF SCP instrument and used a ~28 min gradient Whiper 40SPD method that offers a constant flow of 100 nL/min. Evotips were loaded with K562 (Promega) peptides according to the vendor instructions. Data were acquired in a DIA mode with window placements as shown (Figure 1B). All data were processed using Spectronaut software version 14 with default settings applying a hybrid library.
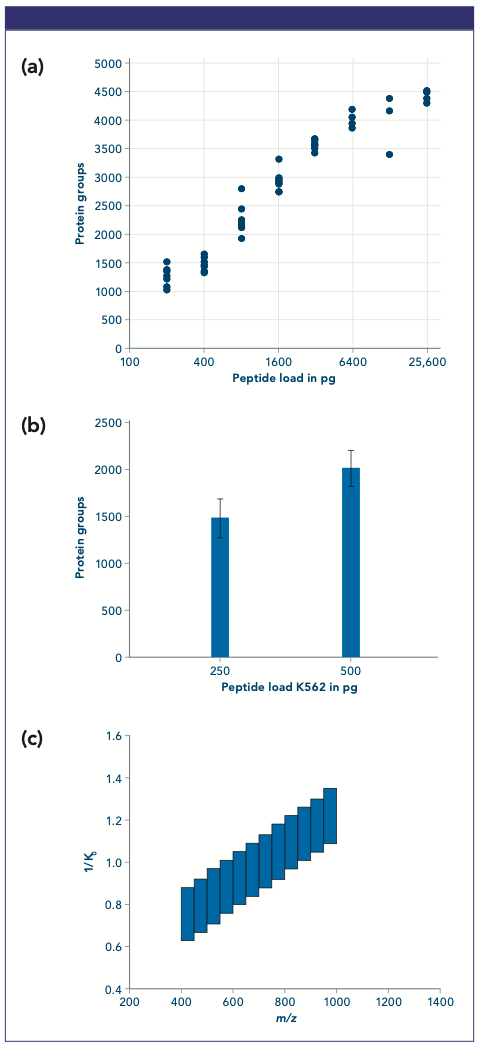
Figure 1: (a) Dilution series of peptides with Whisper 40SPD. (b) Multiple injections of 250 and 500 pg runs using Whisper 40SPD. (c) dia-PASEF® window scheme used for low sample amount.
Results and Discussion
A dilution series of peptide load was performed starting from 200 pg to 25.6 ng in replicates using the ultralow flow method—Whisper 40SPD— from Evosep Biosystems. This method delivers gradient at a flow rate of 100 nL/min further boosting the sensitivity of the platform. About 1200 protein groups could be quantified from the 200 pg loads, and that number increased to an excess of 4000 protein groups for 6.4 ng loads. Then 250 and 500 pg loads, mimicking the amount of peptides resulting from the digestion of one or two isolated cells, were used to test the accessible proteome depth. These samples were analyzed using the Whisper 40 samples per day (SPD) method applying dia-PASEF® methods with a 0.7 cycle time method that covers between 400 and 1000 m/z. The data were processed with a library consisting of 5200 protein groups and about 54,000 peptides. From 250 and 500 pg loads on average 1542 and 2146 protein groups were quantified, respectively.
Work done in the laboratories of Prof. Matthias Mann with the timsTOF SCP combined with robust low flow Evosep One when applied with efficient sample preparation yields exciting results on the biology of the cell cycle (3,4).
Conclusions
- timsTOF SCP provides robust proteome coverage with peptide loads in the range of 250 pg.
- Combination of timsTOF SCP with Whisper methods on the Evosep provide a robust and sensitive platform to perform single-cell proteomics.
References
(1) Z.Y. Li, et al., Anal. Chem. 90(8), 5430–5438 (2018).
(2) F. Meier et al., Mol Cell Proteomics 17, 2534–2545 (2018).
(3) A-D Brunner et al., https://doi.org/10.1101/2020.12.22.423933
(4). A. Mund et al., https://doi.org/10.1101/2021.01.25.427969

Bruker Daltonics GmbH & Co. KG
Bremen · Germany
Tel. +49 (0)421-2205-0
Website: www.bruker.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



