Biopharmaceutical Candidate Screening with Automated Dynamic Light Scattering
Biopharmaceutical Candidate Screening with Automated Dynamic Light Scattering
High-throughput candidate screening is an important pre-formulation step during biopharmaceutical development. Robust pre-screening processes ensure that only candidates with the best therapeutic potential progress to the high-cost stages of development. However, the presence of aggregates and impurities within any of the hundreds of protein samples can misrepresent experimental results and damage the microfluidic channels used during screening. Automated high-throughput dynamic light scattering (HT–DLS) enables rapid and unattended assessment of sample purity to overcome these challenges, maximizing the reliability and productivity of the candidate selection process.
Photo Credit: Jamie Farrant/Getty Images

The biopharmaceutical industry is growing exponentially, with novel biotherapeutic therapies emerging onto the market for a range of disease treatments. The demand for novel biotherapeutics and the extremely high cost of development means that the industry is continually seeking more efficient ways to accelerate the development process. Identifying therapeutic proteins most likely to meet performance and safety targets during early formulation stages ensures that only the best candidates progress towards clinical development, saving time and reducing the risk of costly downstream failure. Two techniques that are particularly powerful in candidate selection are surface plasmon resonance (SPR) and bio layer interferometry (BLI). SPR and BLI enable developers to rank the suitability of early stage candidates by assessing critical properties such as high affinity for the therapeutic target and slow dissociation kinetics. However, their reliability, and the quality of the data generated, is entirely reliant on the purity and solution properties of the analytes, a fact that is often overlooked during method development. Impurities and aggregates can skew the data generated, and also actively block the microfluidic channels that are integral to SPR performance. Evaluating sample purity prior to analysis is therefore an essential precursor to screening. However, with hundreds of samples to analyze, this can be a lengthy, highly expensive, and labour-intensive task. High-throughput dynamic light scattering (HT–DLS) enables the quality of protein samples to be assessed prior to screening without compromising the analytes. Non-destructive measurements of particle size distribution with HT–DLS allows users to quickly assess the presence of aggregates or nanoparticles, preventing much of the uncertainty and productivity loss associated with variable ligand quality. There is also the capacity for automation, meaning that analysis is fast and non-intensive, freeing up expert labour for more valuable tasks. This article explores how the addition of HT–DLS pre-screening to the SPR and BLI process leads to reliable binding data, speeding up and ruggedizing candidate selection.
Ensuring Accurate Screening SPR and BLI are optical techniques that determine the affinity and kinetics of interaction between proteins and their potential binding partners. In high-throughput SPR, active biotherapeutic candidates are immobilized on the surface of a chip while potential binding parent solutions are added at different concentrations. As the analytes flow over the immobilized target molecules within microfluidic channels the best candidates will be strongly attracted to the target on the chip surface. BLI has a similar mechanism of action, except here the target molecules are attached to fibre-optic probes and immersed into protein samples within a microwell plate. The high degree of multiplexing means that BLI offers throughput advantages over SPR. In both methods an optical probe measures variations in the extinction of light and refractive index of the sample at the boundary between the two phases. These signals are proportional to the increase in surface-bound mass and provide information on affinity and kinetics, which directly relates to their suitability for therapeutic benefit. However, the purity of these samples greatly impacts experiment reliability. The evanescent optical fields employed in SPR and BLI extend a few hundred nanometres into the solution. The presence of bulky foreign nanoparticulates or aggregates at this distance from the surface of the chip or fibre probe will cause a spike in signal larger than would be expected of the monomeric-target molecule. Smaller contaminants and aggregates do not generate such noticeable interference in the results; however, their presence can produce a steady stream of small signal fluctuations leading to a degraded optical response.
Figure 1: The possible interactions between aggregates and target molecules in surface plasmon resonance (SPR) and bio-layer interferometry (BLI) screening.
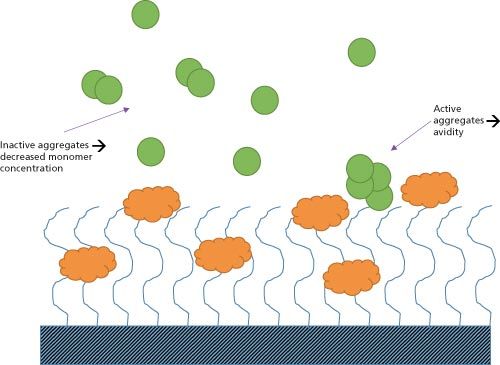
Figure 1 illustrates how inactive and active aggregates lead to incorrect quantification binding properties. Active aggregate clusters have multiple binding sites and interact simultaneously with multiple immobilized molecules, exhibiting a reduced dissociation rate by “hop-scotching” across the surface of the chip. These “avidity” effects lead to affinity being considerably overestimated. In the case of inactive analyte aggregates, effective concentrations are lower than the measured total concentration, causing an apparent decrease in affinity again unrepresentative of the monodisperse sample. Furthermore large aggregates and foreign particulates are especially undesirable in multichannel SPR where the long and narrow fluidic channels are susceptible to blockages from agglomerated proteins or “nanocrud”. Clogging of the microfluidic channels results in the invalidation of lengthy screening processes, which can be extremely costly in terms of wasted run times. It also impacts the instrument, requiring servicing and potentially even replacement of the microfluidic chip. It is therefore essential for researchers to identify poor-quality protein solutions before proceeding with screening. Measuring particle size distribution during quality assessment is an effective method of detecting the presence of aggregates and impurities, and securing sample quality for SPR and BLI processes.
Dynamic Light Scattering Dynamic light scattering (DLS) is a non-invasive, non-perturbative optical technique that measures the size distributions of nanoparticles and proteins in solution across a dynamic size range from less than 1 nm to several microns in size. DLS operates by illuminating a solution with a beam of light. The intensity fluctuations of scattered light relates to the Brownian motion of particles in the system. As the rate of Brownian motion is inversely proportional to particle size, DLS software is able to convert the scattering signal into a particle size distribution for the entire sample. DLS also enables the measurement of an analyte’s diffusion coefficient, which is an important property for identifying mass transfer limitations in certain SPR experiments. Traditionally, DLS is a manual batch measurement technique. Although this has value for certain quality assays, it is not suitable for high volume screening. Fortunately DLS can be performed using high-throughput unattended automation, with sample analysis requiring only 10–30 s per well, including transition times. The absence of fluidics means that there is no potential for sample carryover between the wells, ensuring with a single microwell plate the same levels of performance that can be achieved in batch measurements using hundreds of individual disposable cuvettes, but at much higher throughput and far lower consumable costs. Case Study: Resolution of Protein Quality with HT–DLSMethod: To illustrate the application of automated HT–DLS for rapid sample purity studies a solution screening process was performed on the DynaPro Plate Reader II HT-DLS systems using Dynamics data handling software (Wyatt Technology). The software provides automated analysis and visualization of DLS results, often as a heat map indicating good, intermediate, and poor protein quality. This enables users to evaluate samples to determine whether solutions are suitable for SPR microfluidics and whether the sample is pure enough to produce accurate SPR or BLI measurements. The 96-sample well plate (Corning) was filled with protein samples and the software was configured to resolve the data as a heat map based on previously defined poor, intermediate, and high quality particle size distributions. Figure 2 summarizes the data output of this process.
Figure 2: Visualization of protein quality as a heat map. Total data acquisition time for 96 wells was < 45 min. Data courtesy of Sabin Vaccine Institute and Texas Children’s Hospital Center for Vaccine Development, Baylor College of Medicine.
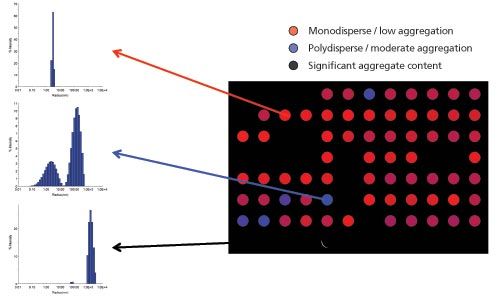
Results: The sample wells marked as red show a single, narrow distribution profile peak at a size corresponding to that of the monomeric analyte. These samples are therefore likely to be classified as high-quality and will be allowed to proceed to the binding assay with confidence. In contrast, samples within the blue wells demonstrate a broader monomeric peak, indicating the presence of some oligomers and low levels of foreign particles tens of nanometers in size. These are then classified as being of intermediate quality and either discounted or flagged as a potential source of inaccurate results. Finally samples within the black cells exhibit substantial particulate content in the micron-size range. This indicates that the samples have either been contaminated during handling or are particularly prone to aggregation. Either way, identifying these impurities at an early stage allows the system to remove these samples from the screening process, preventing them from continuing on to the binding assay. The automated run was performed in just 45 min, without any drop off in sensitivity compared with manual batch operation. These fast measurements allow confidence in sample selection for SPR and BLI testing, reducing the uncertainty and any potential loss in productivity arising from variable ligand quality. Conclusion As the biopharmaceutical development pipeline progresses, the cost of candidate failure rises exponentially. Employing SPR or BLI techniques to select the candidate molecules most likely to meet therapeutic targets is an effective way to mitigate these risks. Particle size distribution measurements using HT–DLS play an important role in both SPR and BLI, ensuring that the samples analyzed are high quality and that the data is truly representative of analyte-ligand interactions. This reduces the risk of costly downstream failure as well as preventing microfluidic channel clogging. Automated HT-DLS can be seamlessly integrated into existing screening workflows to provide classification on the quality of solutions. Dr. Daniel Some, Ph.D. is Principal Scientist and Director of Marketing at Wyatt Technology Corporation. As Director of Marketing, Dr. Some is responsible for conveying to customers the benefits of Wyatt’s products and services, as well as learning about specific needs and opportunities expressed by customers. As Principal Scientist he investigates techniques for characterization of macromolecular interactions, contributing to the development of new instruments and applications. Dr. Some obtained his B.Sc. from the Israel Institute of Technology and Ph.D. from Brown University, both in Physics, and completed his post-doctoral study at Los Alamos National Laboratory and Weizmann Institute of Science. Prior to joining Wyatt Technology he worked on R&D of semiconductor wafer inspection tools and defense electro-optics. E-mail: dsome@wyatt.com
Website: www.wyatt.com This article was first featured in LCGC's digital magazine, The Column. Click here to view the full issue>>

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.














